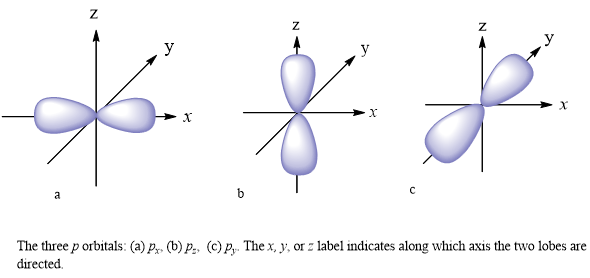P Orbital
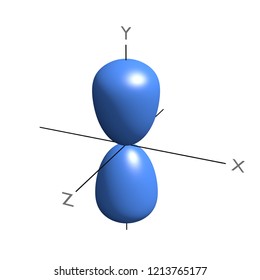
The one shown below points up and down the page.
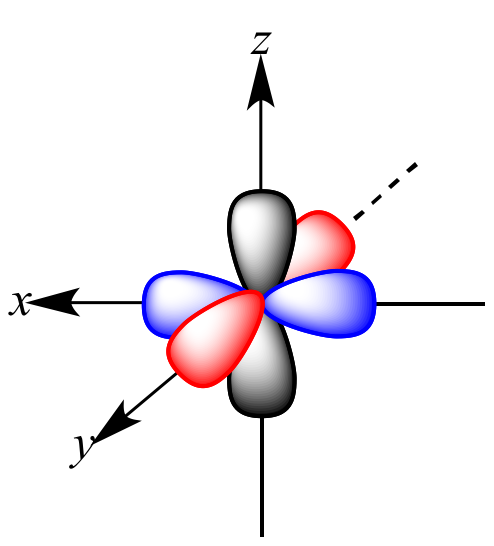
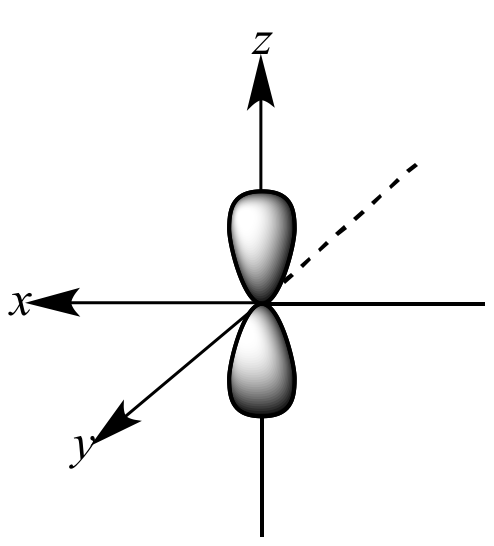
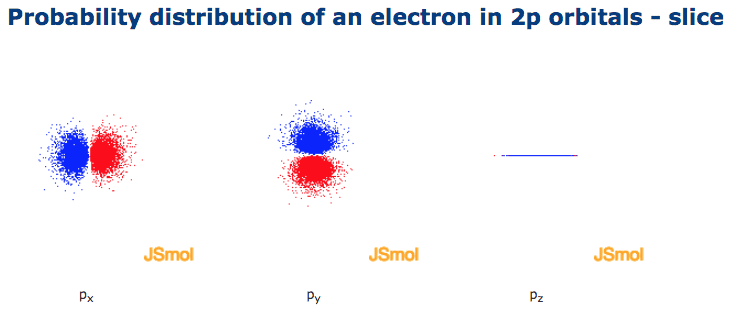
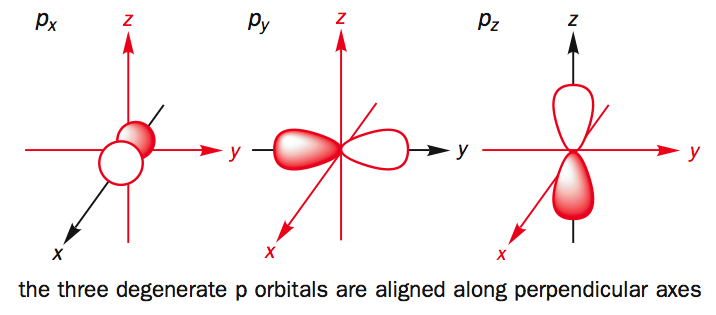
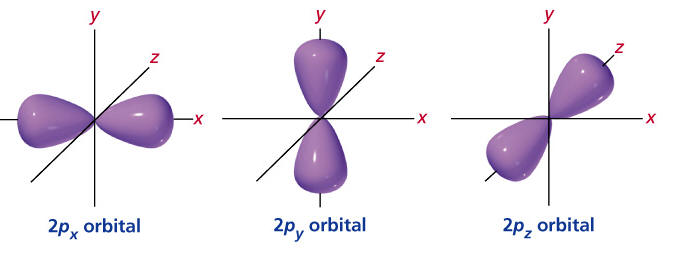
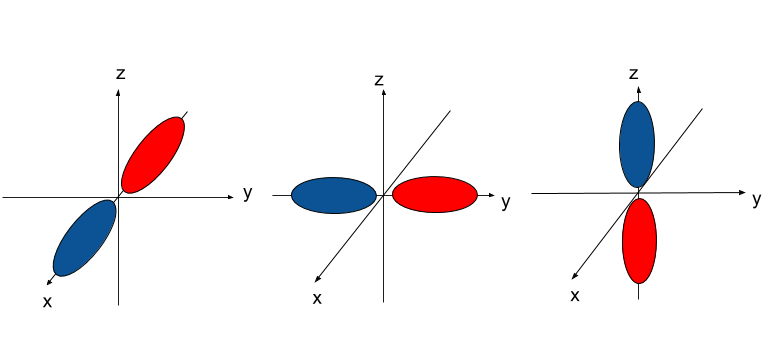
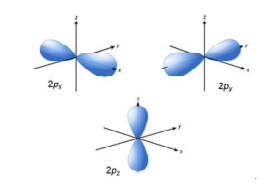
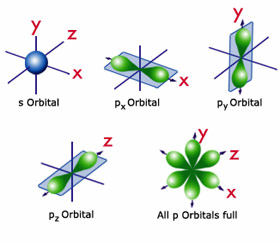
P orbital. One p orbital can hold a maximum of 6 electrons. The node of the dumbbell occurs at the a tomic nucleus so the probability of finding an electron in the nucleus is very low but not zero. These orbitals are designated as p x p y p z orbitals. Specifies the orientation in space of an orbital of a given energy n and shape l.
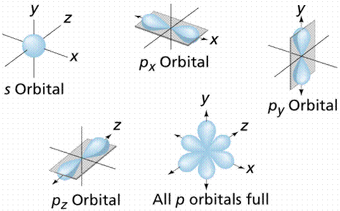
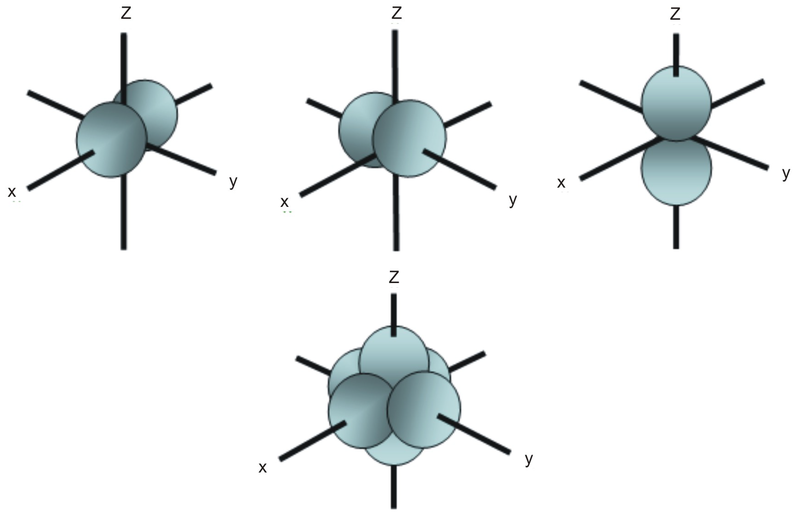
The letter p stands for principal it describes the angular momentum of electrons in the p orbital. The three p orbitals are at right angles to each other and have a lobed shape. Unlike an s orbital a p orbital points in a particular direction.
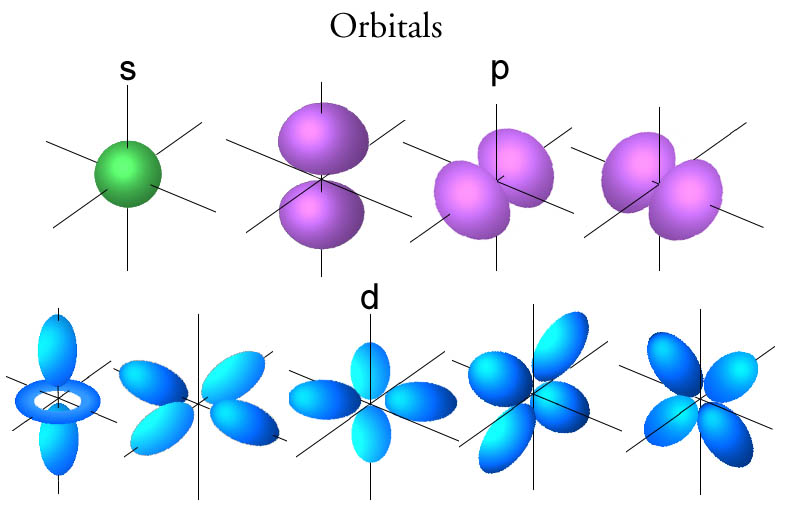
These orbitals are similar to the p orbital shape but with more petals like a cloverleaf. These names together with the value of n are used to describe the electron configurations of atoms. They can also have ring shapes around the base of the petals. These electrons occupy subatomic orbitals.
Click the images to see the various views. P orbital is an atomic orbital having a dumbbell shape. Not all electrons inhabit s orbitals. However at the second level there are also orbitals called 2p orbitals in addition to the 2s orbital.
The second ℓ 1 is called a p orbital. Consists of three orbitals called p orbitals. The p orbital is a dumbbell shaped or lobed region describing where an electron can be found within a certain degree of probability. The individual orbitals are labeled with the magnetic quantum number ml which can take the 2 l 1 values l l 1 l.
The size of the p orbitals also increases as the energy level or shell increases. At the first energy level the only orbital available to electrons is the 1s orbital. The simple names s orbital p orbital d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ 0 1 2 and 3 respectively. P orbitals have a higher energy than that of s orbitals.
And a d subshell l 2 consists of five orbitals called d orbitals. The orbital occupied in the. The orbital of an electron shell in an atom in which the electrons have the second lowest energy. Thus the s subshell has only one orbital the p subshell has three orbitals and so on.
The p sub shell can hold a maximum of six electrons as there are three orbitals within this sub shell. This number divides the subshell into individual orbitals which hold the electrons. There are 2 l 1 orbitals in each subshell. What is p orbital.
P orbitals are usually polar and form a teardrop petal shape with the point towards the nucleus. P orbitals have two lobes directed on opposite sides of the nucleus.