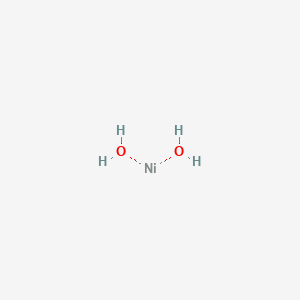
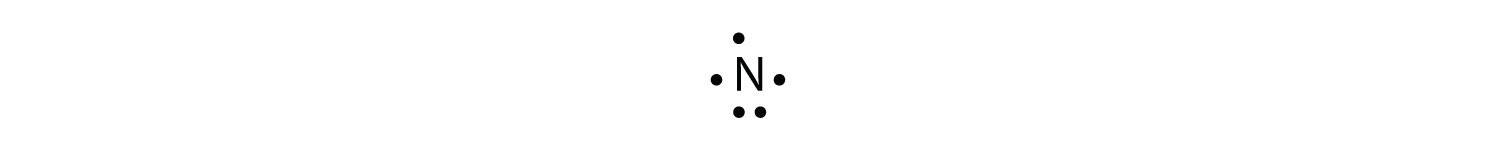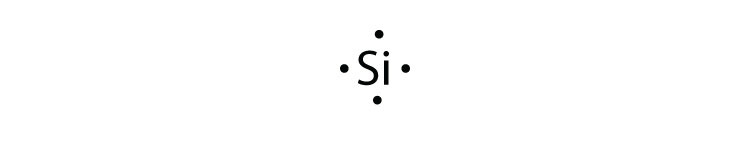Ni Lewis Dot Structure

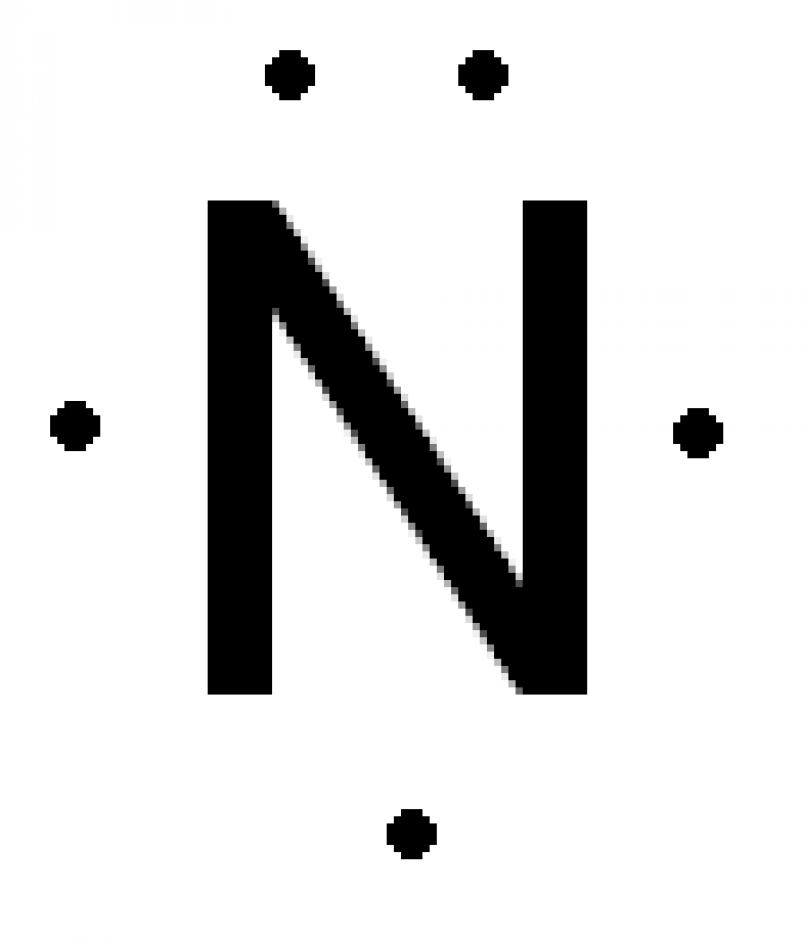
Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired.
Ni lewis dot structure. For the i structure use the periodic table to find the total number of valence electro. Then determine whether the atoms are held together by a single double or triple bond. For example use 1 line to show a single bond or draw 2 lines if they have a double bond. For the h lewis structure use the periodic table to find the total number of valence electrons for the h atom.
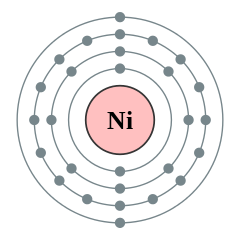
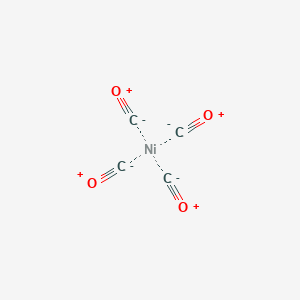
It wold just be the atomic symbolsurrounded by it s 10 valence electrons dots. Once we know how many valence electrons there. A step by step explanation of how to draw the no lewis structure nitric oxide. So for example the electron configuration of nickel would be ar 4s2 3d8 yes technically this is a noble gas configuration but i want to speed things up and so it would have two valence electrons based on the two electrons it holds in its last shell 4s since 4 is bigger than 3.
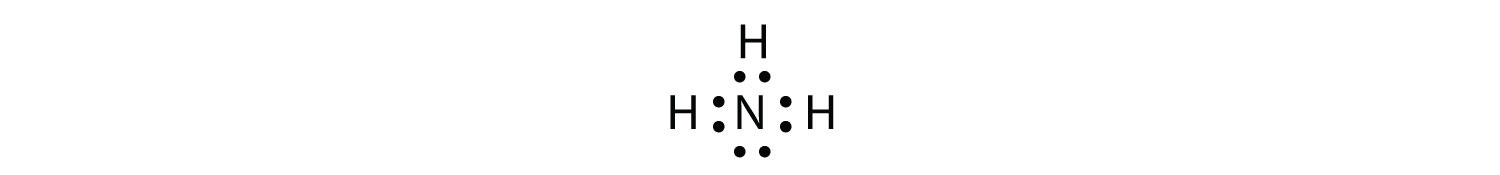
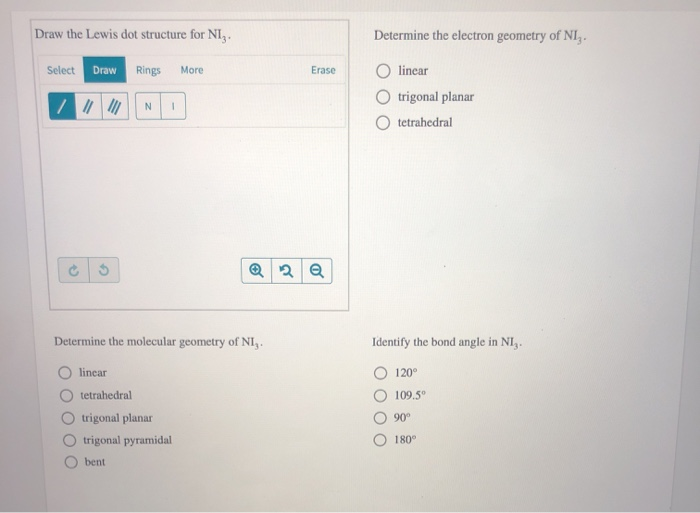
A pair of dots between chemical symbols for atoms represents a bond. Nickel is an unbonded element. For the no lewis structure you have an odd number of valence electrons. Put the n in the middle with three k s around it such as one on each side and 1 below.
Put the 8 dots all of the n and put 1 charges on all k s but a 3 charge on the n. Alternate mg and p like mg. Next draw lines between the atoms to represent that bond. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as electron bookkeeping.
What is lewis dot structure for nickel answers. A step by step explanation of how to draw the i lewis dot structure. Finally you ll understand all those weird pictures of molecules with the letters and the lines and the dots.