N Electrons Are Present In

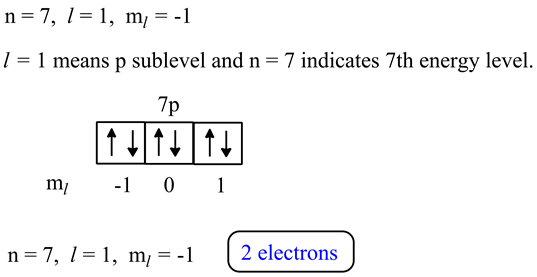
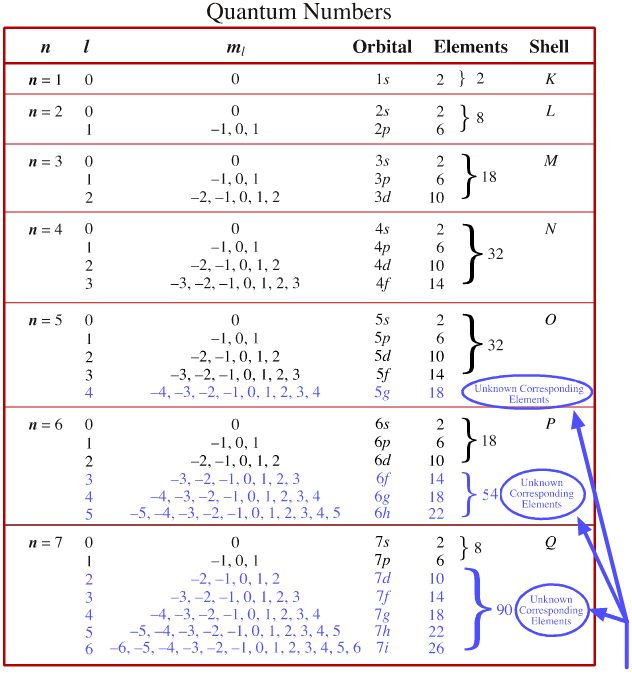
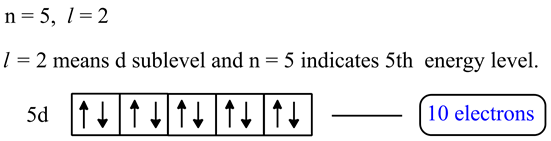
For the 5s orbital n 5 and l 0 therefore n l 5.
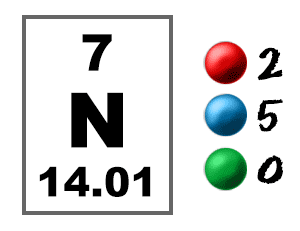
N electrons are present in. 2 the principal quantum number n describe the size of the orbital. Electrons fill the sublevels in energy order 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p. They are atoms of n 15 an isotope of nitrogen. Note some principal energy levels start to fill before previous ones finish.
16 3 as a mixed number. Therefore it has gained 3 electrons compared to the neutral state. Dot structures make it easy to count electrons and they show the number of electrons in each electron shell. When an ion has a negative charge the atom has gained electrons.
In many cases this will be the same number as electrons but this is not guaranteed. Hence we can conclude that the ion has 7 protons and 10 electrons. Many nitrogen atoms have seven neutrons 14 7 7. Written configurations require minimal space and show the distribution of electrons between subshells.
To calculate the total number of present electrons you simply add the amount of extra charge to the atomic number. For example atomic number of nitrogen is 7 and in a ion there will be 7 3 10 electrons and there will be 7 protons. An s subshell has only one orbital and as an orbital can hold a maximum of two electrons therefore the maximum number of electrons that would be present in the 5s orbital would be 2. This means a neutral atom of nitrogen must have seven electrons to match its seven protons.
Although most elements have the same number of electrons and protons in their atoms an element can lose or gain electrons as part of a chemical reaction. Nitrogen s atomic number is 7 therefore this ion has 10 electrons. In the case of a negative ion there are fewer protons than electrons. Look at the oxidation number if necessary.
For example n 3 has a 3 charge. In a neutral atom the number of protons must equal the number of electrons. Answer number of electron fit in the orbital n 3 and l 1 is 6total total number of electron 6 some important information 1 n denote the principal quantum number. Arrow and line diagrams show the spin of electrons and show every orbital.
Some atoms of nitrogen however have eight electrons. For the 4p orbital n 4 and l 1 therefore n l 5. Students are also searching for. Ex 4s fills before 3d because 4s has less energy than 3d.
Which statement is true about bf3 a nonpolar molecule. It must fill first.