3 3 Dose Escalation

The 3 3 design is the most common choice among clinicians for phase i dose escalation oncology trials.
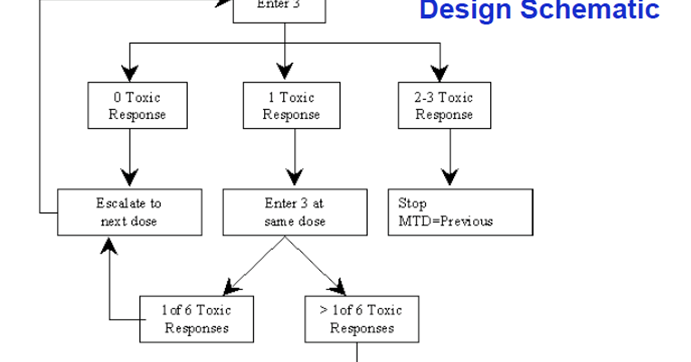
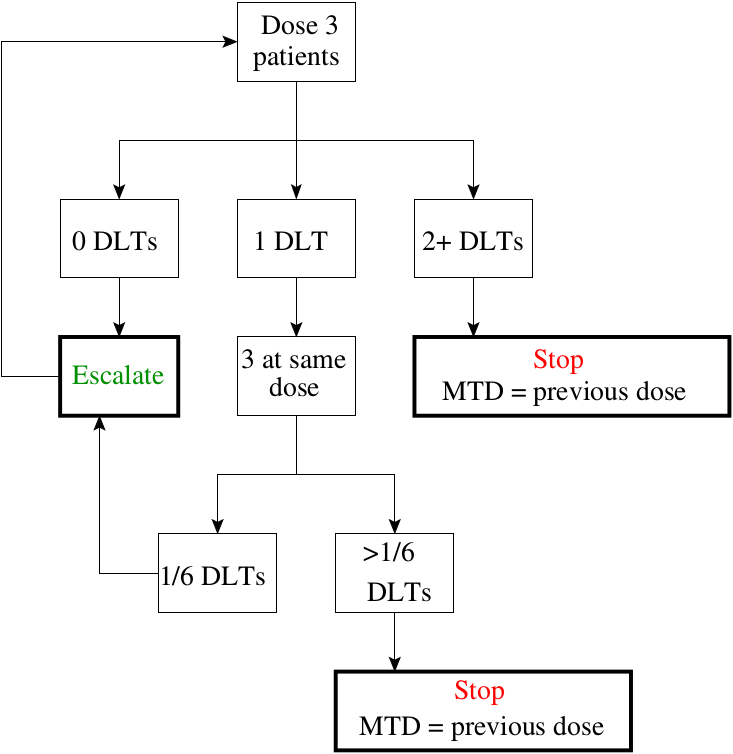
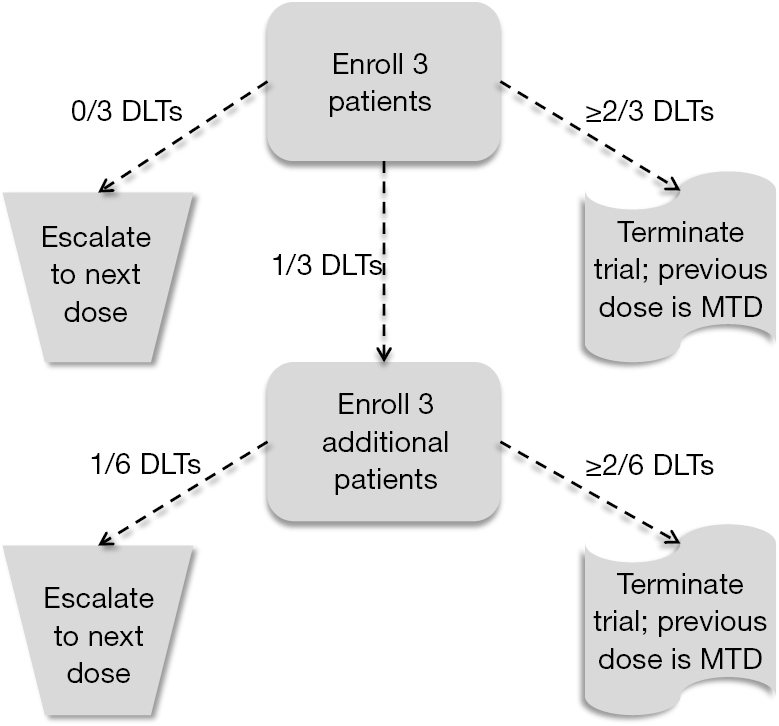
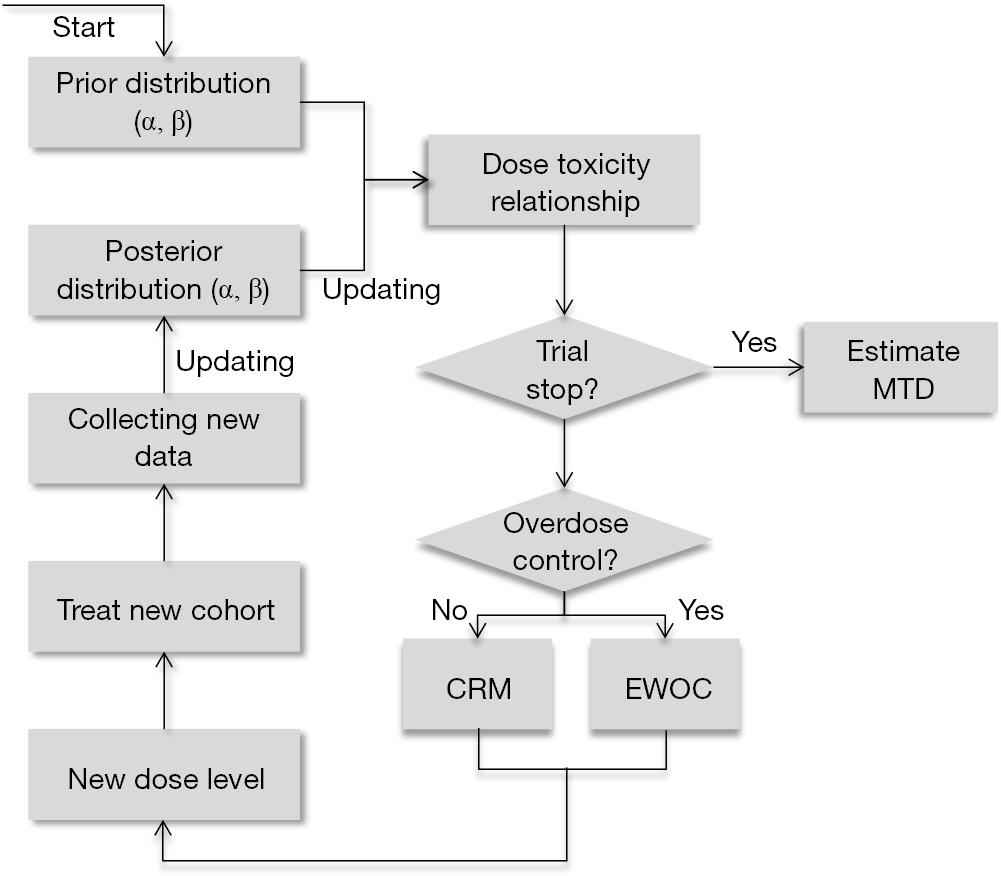
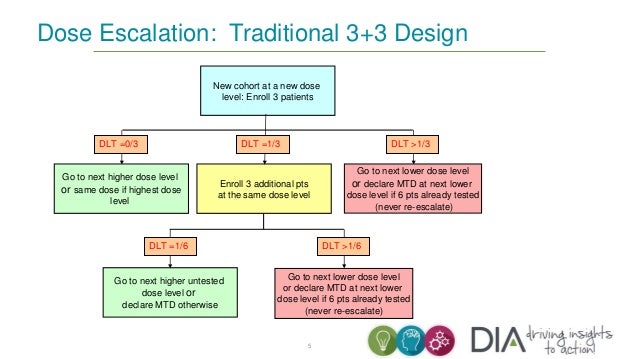
3 3 dose escalation. The 3 3 is conservative with respect to the number of toxicities which occur because the dose escalation is performed with caution but it can potentially lead to a large number of patients needed to estimate the mtd especially if the true mtd is located in the upper range of the doses tested. One of the benefits of the 3 3 design and likely a major factor to its popularity is that it is very simple and can be easily understood by study investigators and clinical researchers. However several concerns have been raised about the quality of the operating characteristics of the 3 3 design. 15 3 1 3 toxicity induced modifications in enrollment or dosing safety.
The protocol specified 100 mg m. The traditional standard dose escalation schedule in the development of cancer therapeutics uses the so called 3 3 design to avoid selection of a phase 2 clinical trial dose that causes a treatment limiting toxicity in more than 17 of subjects a standard considered acceptable as an outpatient therapeutic for patients with limited options and life threatening diseases. We addressed the ability of dose escalation to determine the mtd or the bad in trials that used a dose escalation design. The rate of grade 3 4 vaccine related systemic toxicities was 1 25 adverse events per 100 patients and 2 per 1 000 vaccines.
The conventional 3 3 design as described by storer1in 1989 was originally introduced in the 1940s2and was among the earliest dose escalation and de escalation schemes utilized. Of ender g twice a day for 4 weeks administered intravenously for the first cohort. If one dlt is observed among the previous three patients three more patients are treated at this dose. In recent reviews more than 95 of phase i trials have been based on the 3 3 design.
3 1 2 dose escalation and maximum dose and duration in phase 1. Successive cohorts were given doses of 125 and 150 mg m. If no dose limiting toxicities dlts are observed the trial proceeds to the next higher dose with three new patients. The traditional 3 3 dose escalation design starts off by treating three patients at a conservative low dose.
A traditional 3 3 dose escalation design will be implemented. Given that it is intuitive and its implementation does not require a computer program clinicians can conduct 3 3 dose escalations in practice with virtually no logistic cost and trial protocols based on the 3 3 design pass institutional review board and biostatistics reviews quickly. A traditional 3 3 dose escalation design was implemented. Successive cohorts of participants 3 participants cohort were each started on a fixed dose of ender g.