3 3 Design

Start by allocating lowest dose level to first cohort.
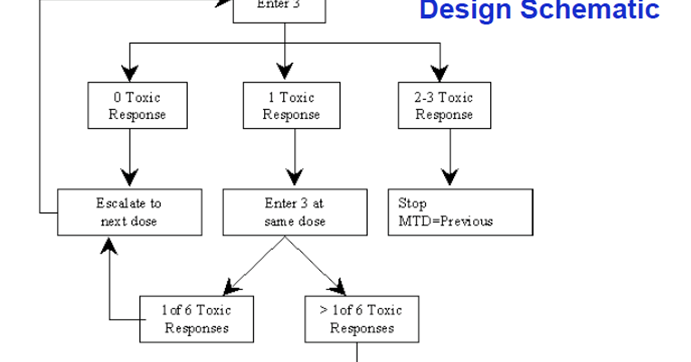
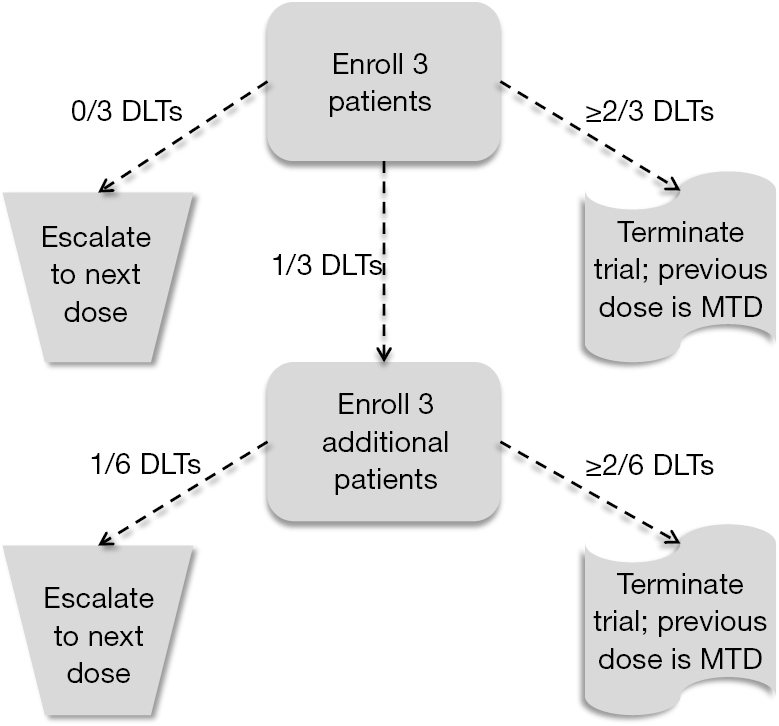
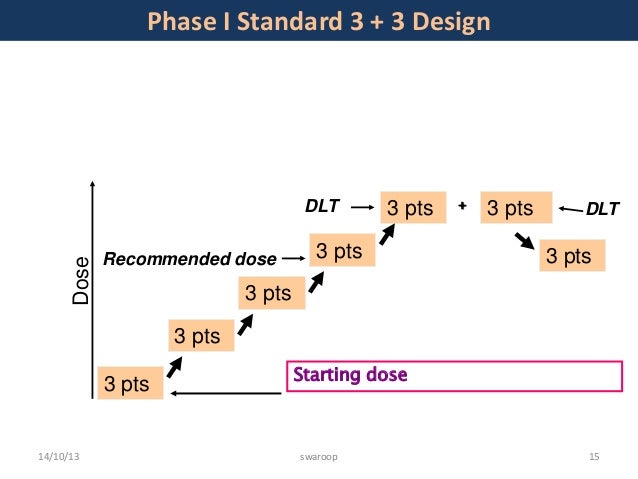
3 3 design. In summary the 3 3 design is. Repeat until mtd obtained or trial is stopped. 2 escalate the dosage if no toxicity is observed in all 3 patients. According to this design it proceeds with cohort of 3 patients who are treated at a starting dose that is considered to be safe based on extrapolation from animal toxicological data.
The 3 3 design is very straight forward robust simple and can be very well understood by clinicians and investigators. 3 if 1 6 patients has toxicity escalate. Otherwise an additional 3 patients are treated at the same dose level. Adaptively escalate de escalate based on observed dlts.
Use an adaptive bayesian method to identify a dose with the targeted dlt rate. The traditional rule based design 3 3 has been shown to be less likely to achieve the objectives of dose finding trials when compared with model based designs. Predefine dose levels for escalation as if for a 3 3 design. In other words the target toxicity rate of the 3 3.
We propose a new rule based design called i3 3 which is based on simple but more advanced rules that account for the variabilities in the observed data. If more that 2 6 patients have toxicity use the lower dose as the mtd. If 2 6 patients have toxicity declare the current dose as the maximum tolerable dose mtd. The 3 3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt.
3 3 design is the most commonly used design in conducting phase i cancer clinical trials.