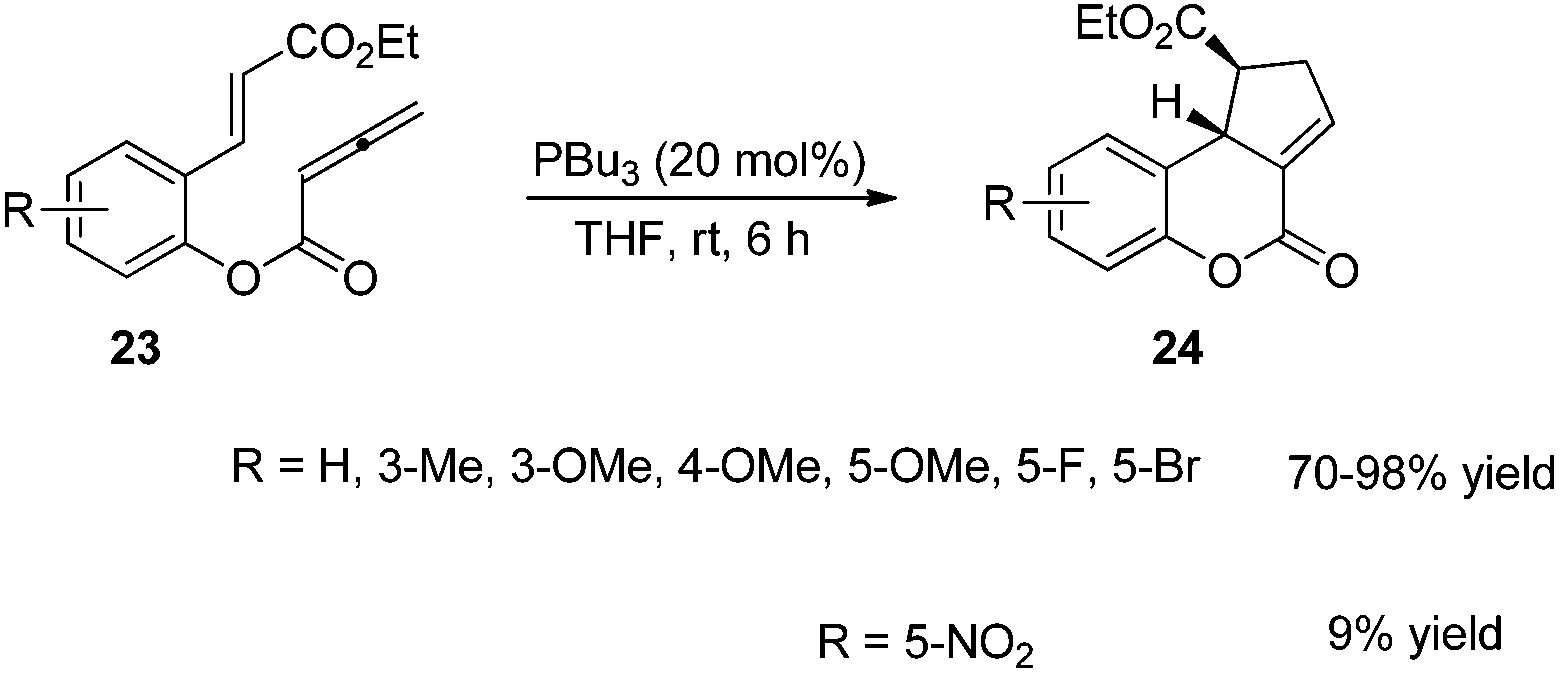
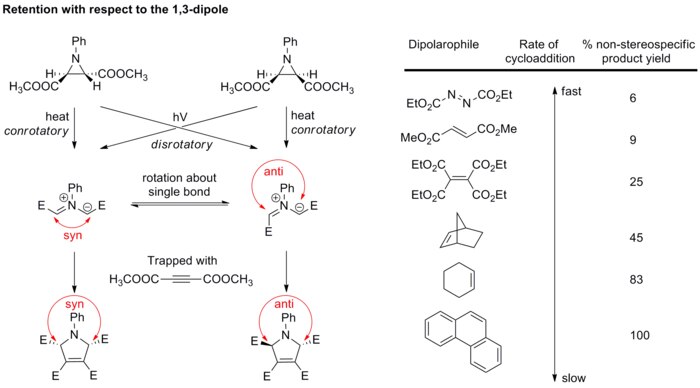
3 2 Cycloaddition
Access to 1 3 disubstitued 1h 1 2 4 triazoles issa yavari a and omid khaledian a.
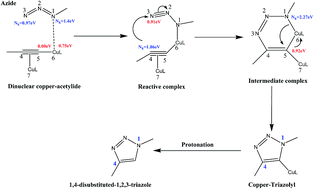
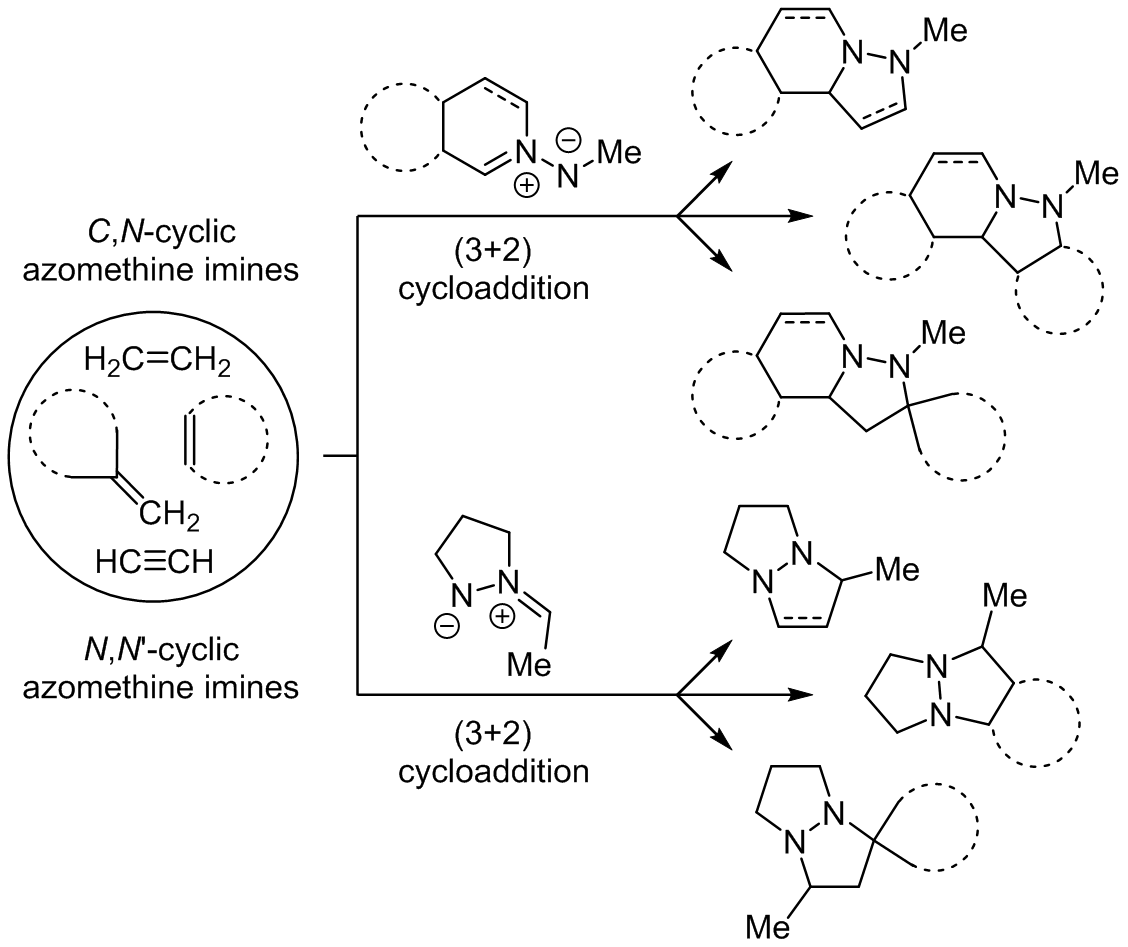
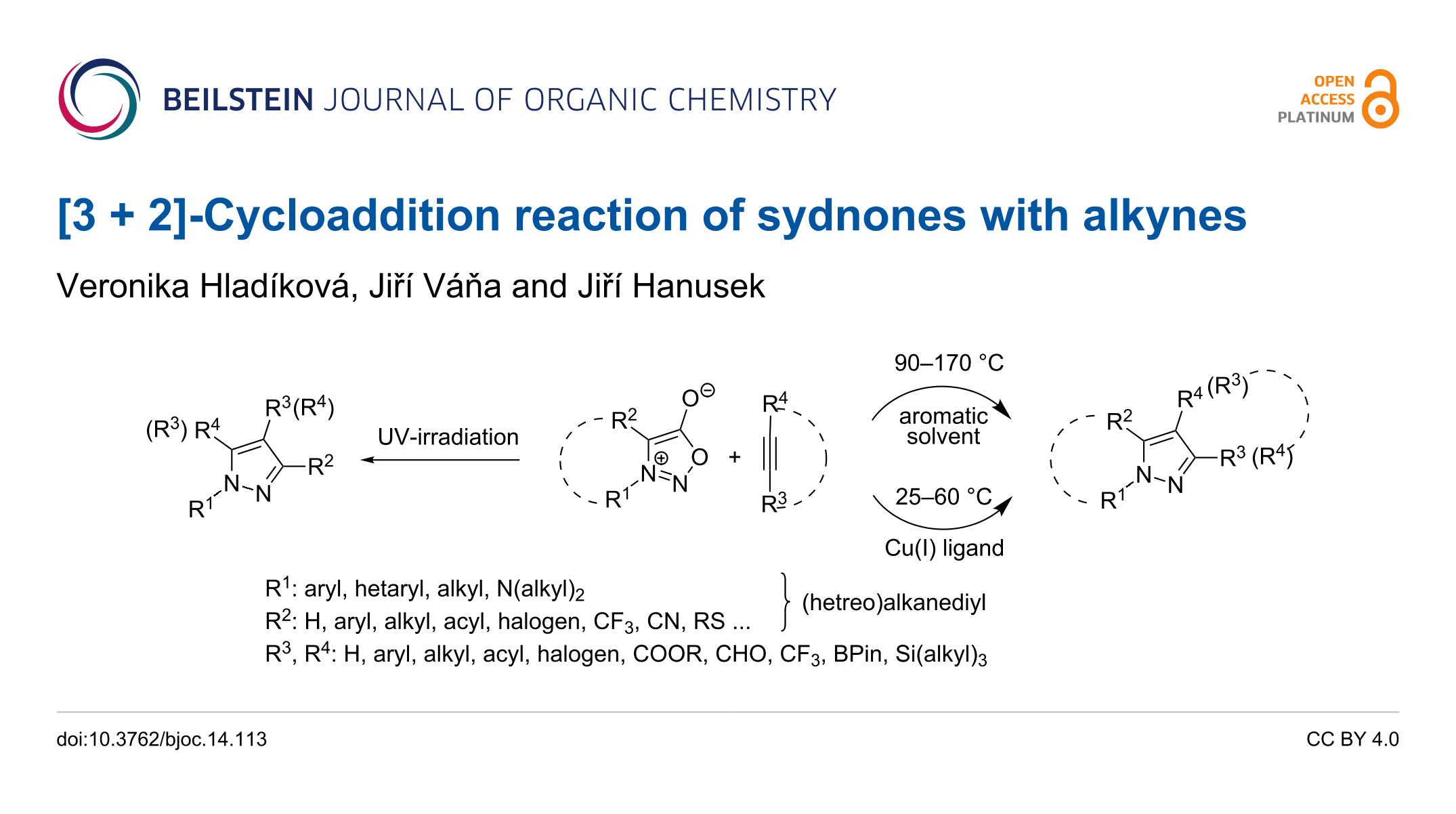
3 2 cycloaddition. Xiyan lu s group many research groups expanded its substrate scope and developed its asymmetric variants and demonstrated its synthetic applications as well. By atom convention the diels alder reaction is a 4 2 cycloaddition producing a six membered ring 1 3 dipolar cycloaddition is a 3 2 cycloaddition giving a five membered ring and cycloaddition of butadiene with an allyl cation is a 4 3 cycloaddition forming a seven membered ring. Hence the reaction is sometimes referred to as the huisgen cycloaddition this term is often used to specifically describe the 1 3 dipolar cycloaddition between an organic azide and an alkyne to generate 1 2 3 triazole. Krasavin synthesis 2019 51 3998 4005.
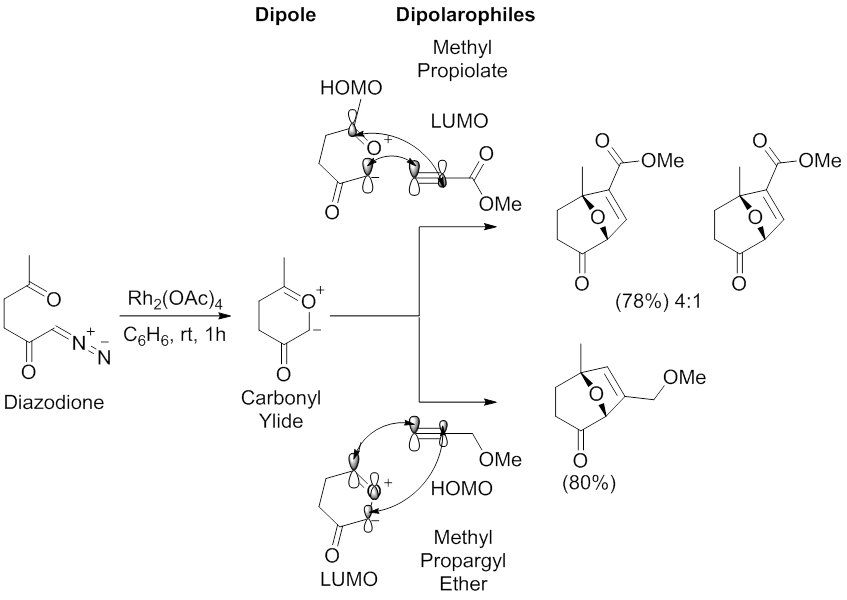
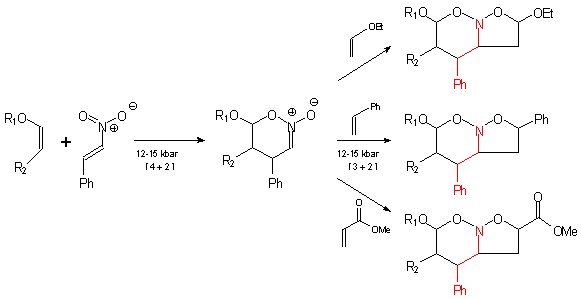
The dipolarophile is typically an alkene or alkyne but can be other pi systems. A formal 3 2 cycloaddition reaction of n methylimidazole as a masked hydrogen cyanide. A diastereoselective 3 2 dearomative annulation of 3 substituted indoles with α haloketones has been developed. Nitrone 3 2 cycloaddition xylene δ 3 2 cycloreversion n o co2me n me co2me o o ph cocaine n oco 2me n o co2me 1.
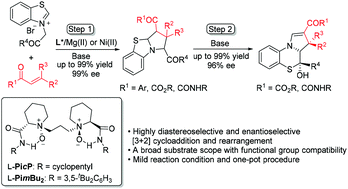
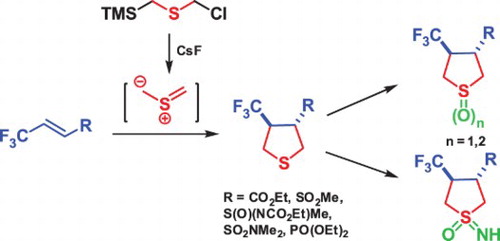
1 3 dipolar cycloaddition is an important route to the regio and stereoselective synthesis of five membered heterocycles and their ring opened acyclic derivatives. In this system the standard diels alder reaction is a 4 2 cycloaddition the 1 3 dipolar cycloaddition is a 3 2 cycloaddition and cyclopropanation of a carbene with an alkene a 2 1 cycloaddition. Bzcl 66 overall meo2c. 3 2 cycloaddition of α diazocarbonyl compounds with arenediazonium salts catalyzed by silver nitrate delivers 2 5 disubstituted tetrazoles s.
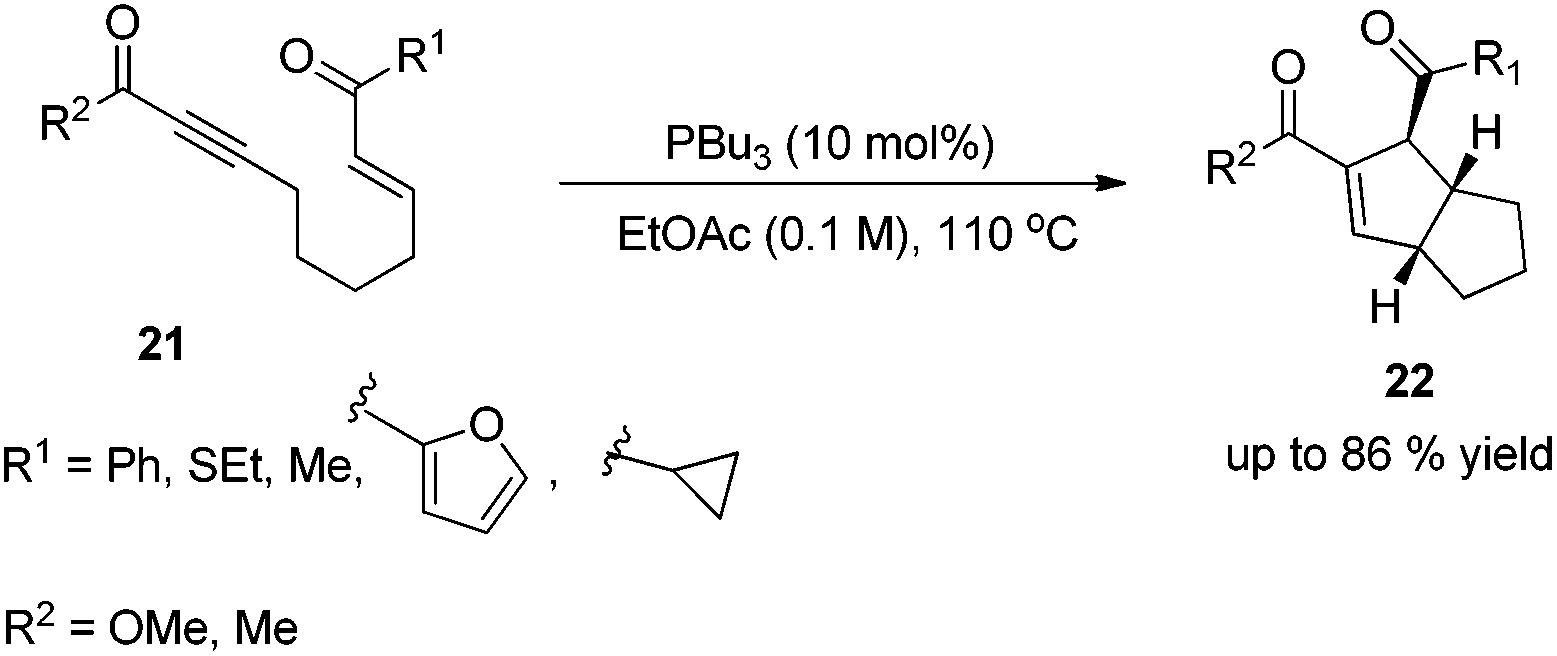
This methodology provides easy access to highly functionalized cyclopenta or cyclohexa fused indoline compounds which are common structures of many natural products. Significant regiochemical control was observed. When r is an electron donating group alkyl or aryl the dominant fmos are the homo of the dipolarophile and the lumo of the nitrone. Abstract the 3 2 cycloaddition reaction involving oxyallyl cations has proven to be a versatile and efficient approach for the construction of five membered carbo and heterocycles which are prevalent frame works in natural products and pharmaceuticals.