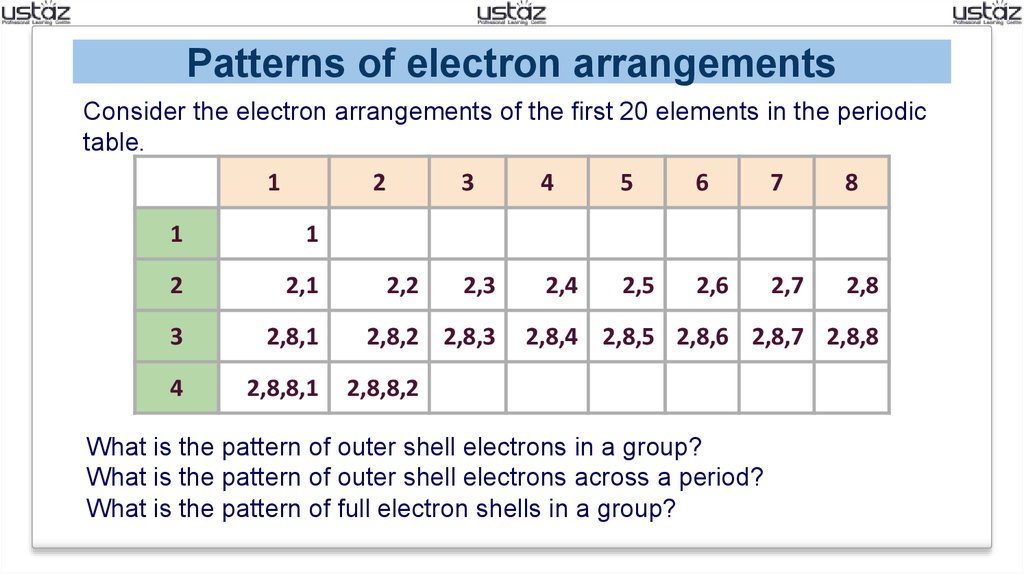
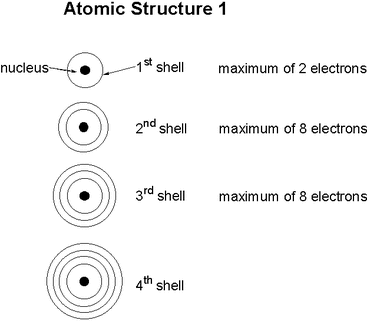
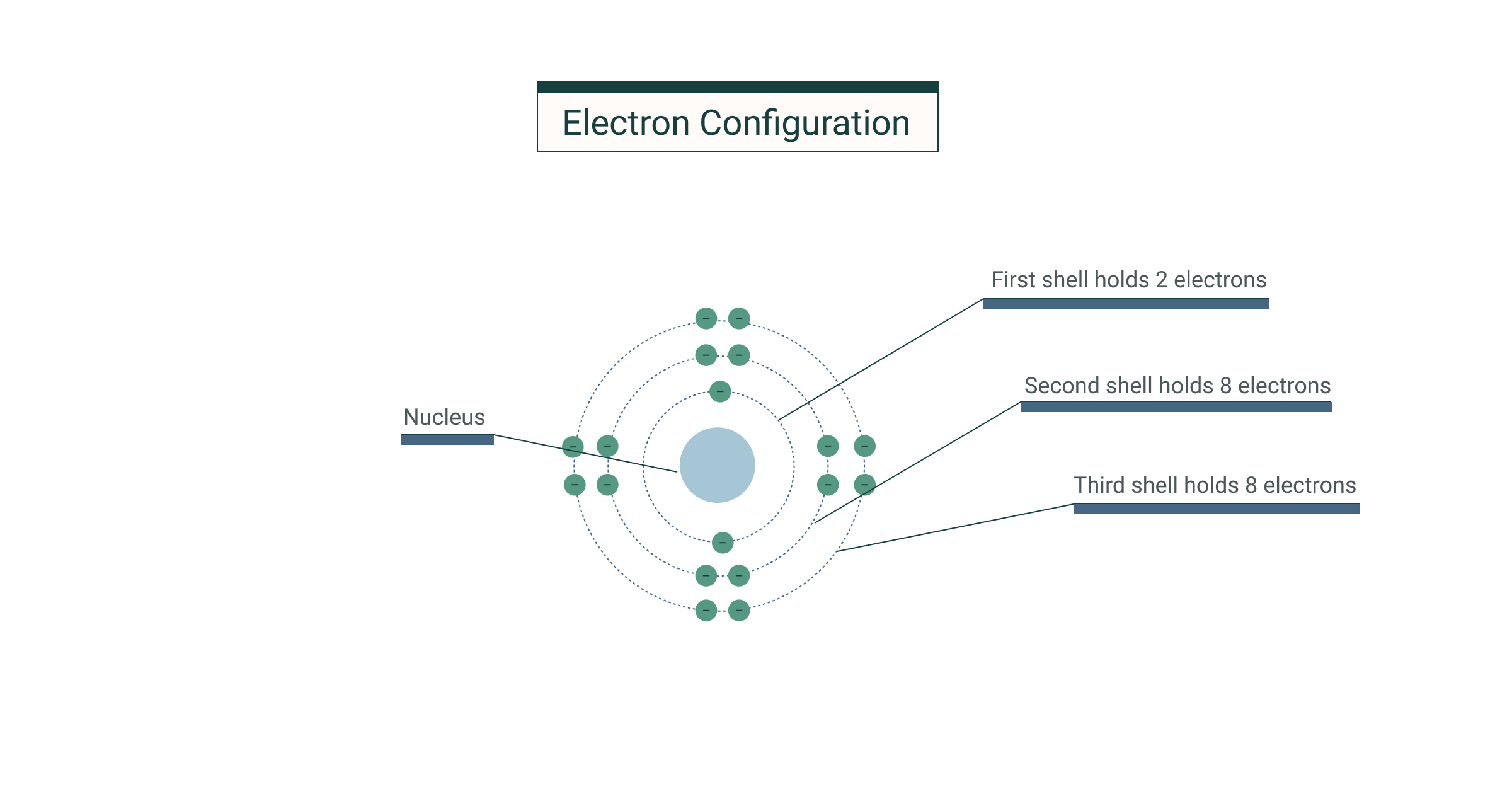
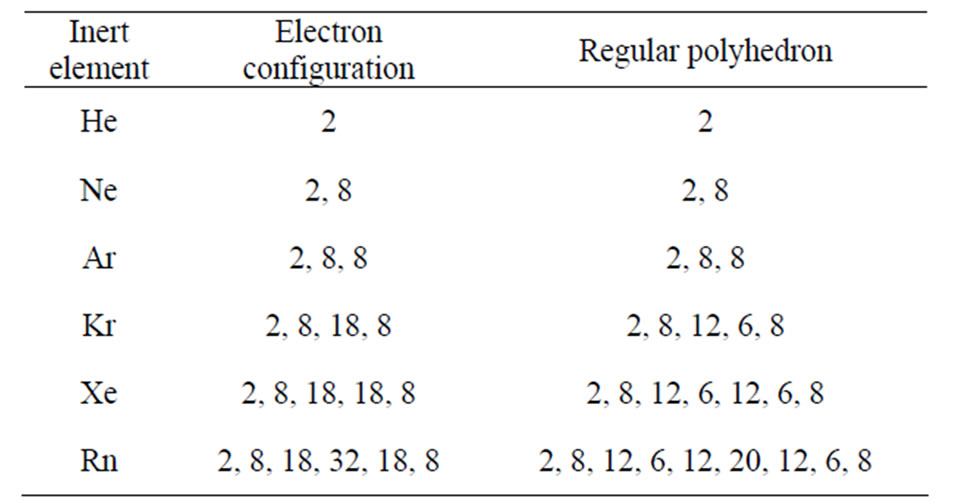
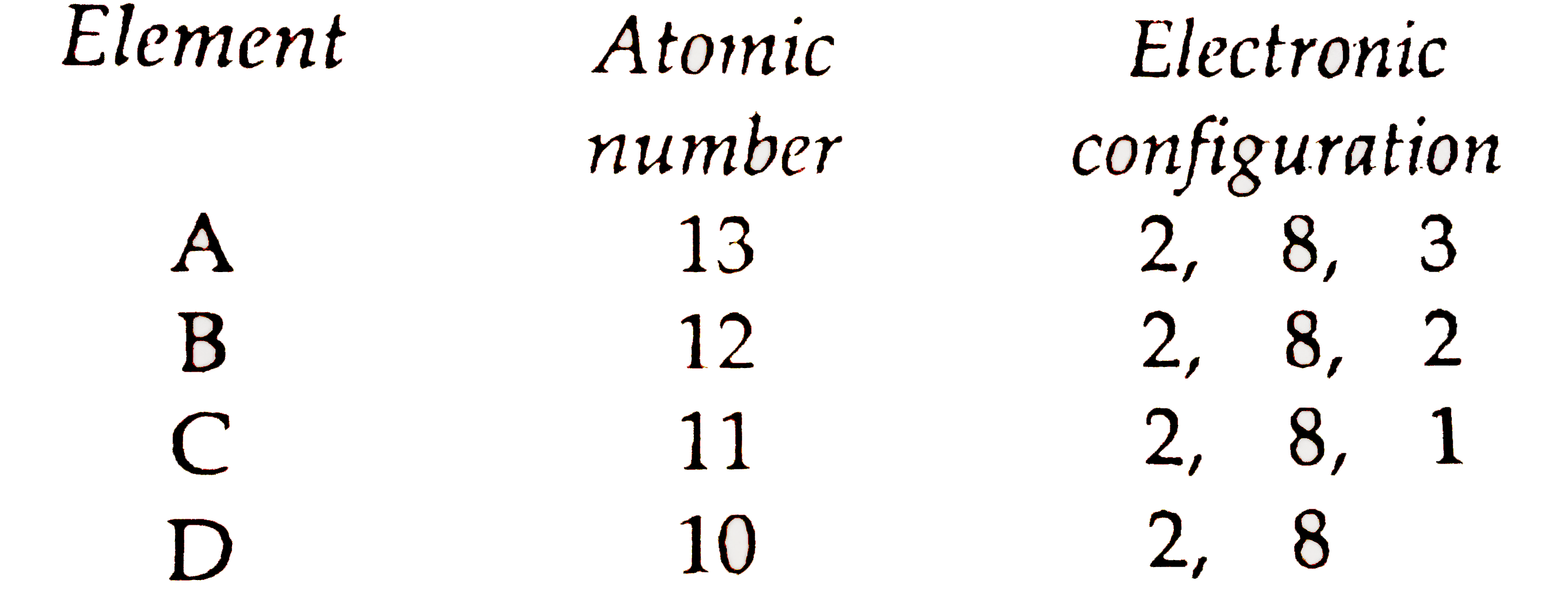2 8 8 2 Electron Configuration

Elements with stable noble gas configuration are referred to as inert.
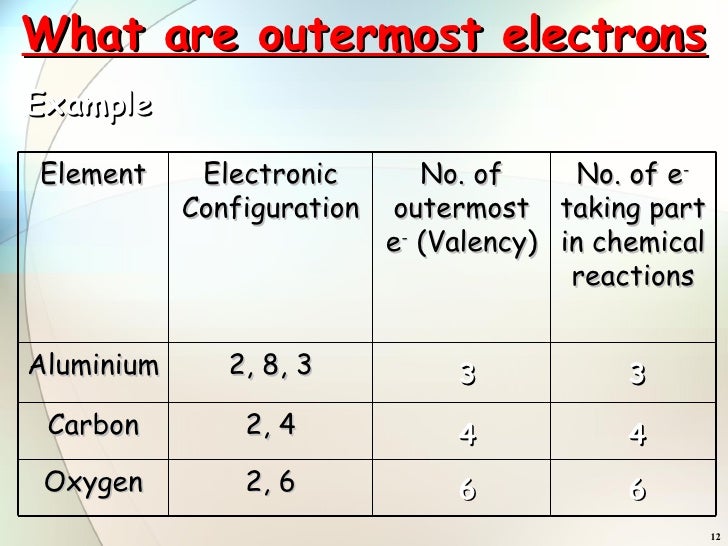
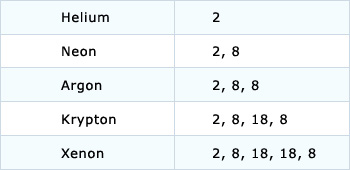
2 8 8 2 electron configuration. Argon has the atomic number 18 and is the element that has the electron configuration 2 8 8. The electron configuration is ne 3s 2 3p 3. This gives a valence electron configuration of 3s 2 3p 3. An overview of the role of orbitals in electron configurations and how to write electron configurations.
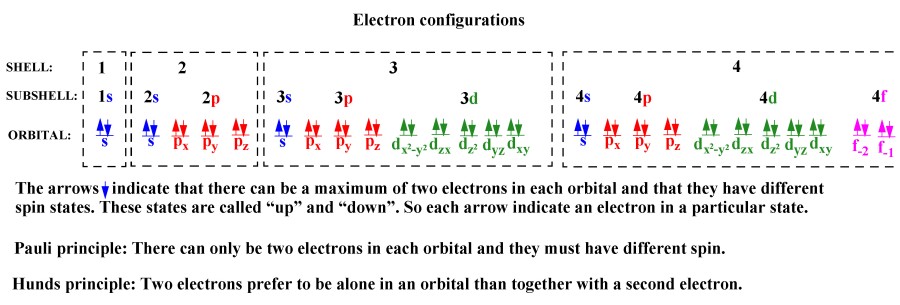
C we obtain the valence electron configuration by ignoring the inner orbitals which for phosphorus means that we ignore the ne closed shell. The relative energy of the subshells determine the order in which atomic orbitals are filled 1s 2s 2p 3s 3p 4s 3d 4p and so on. Electron configurations and orbital diagrams can be determined by applying the.