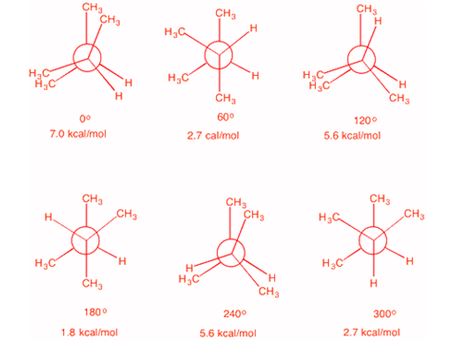
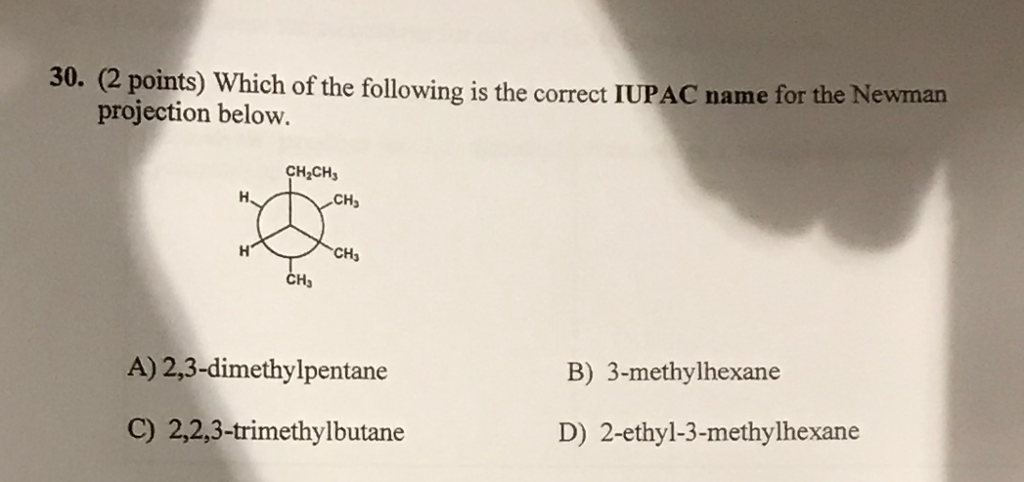
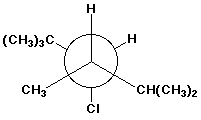
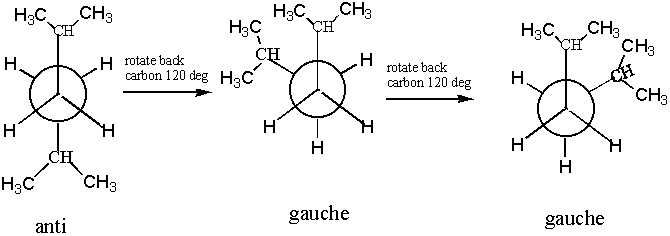
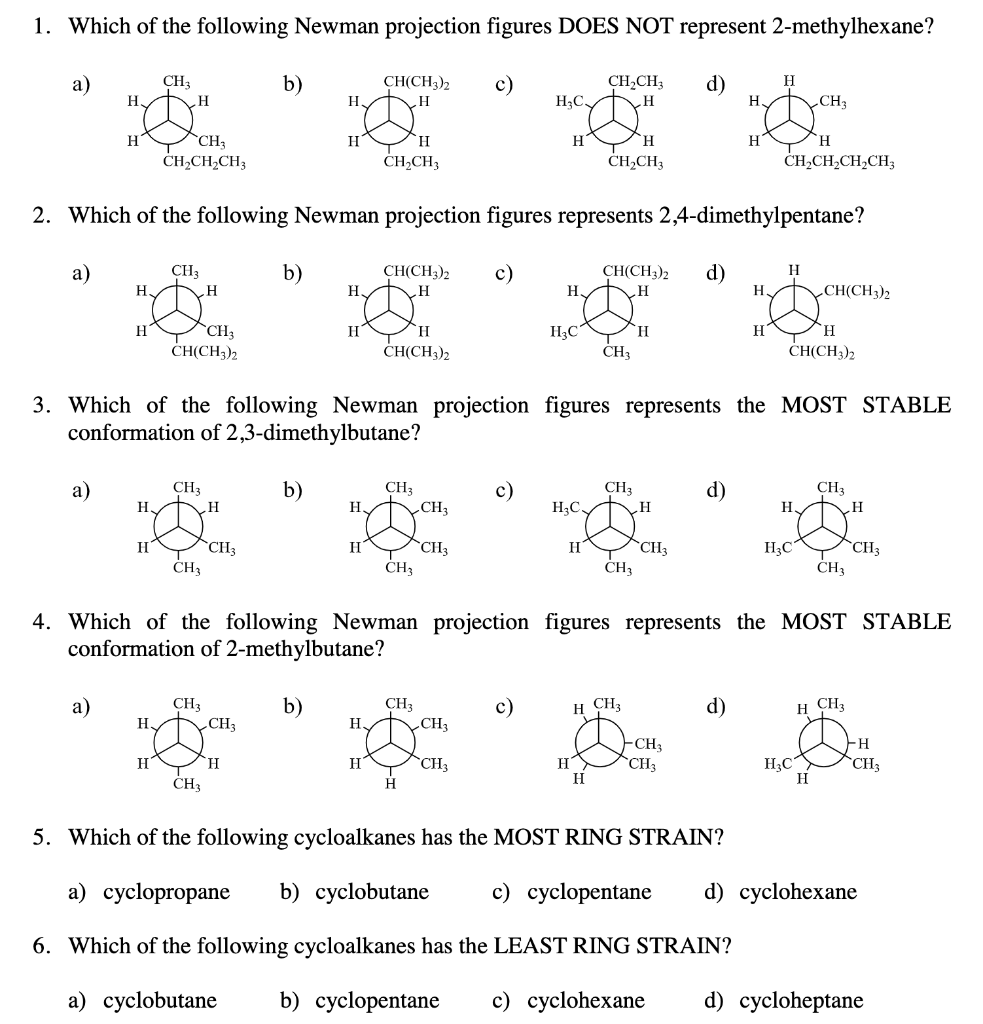
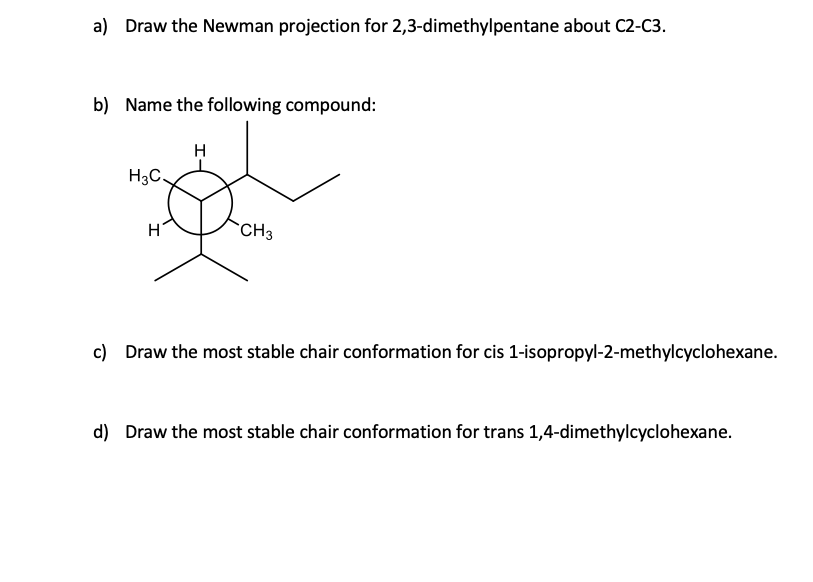
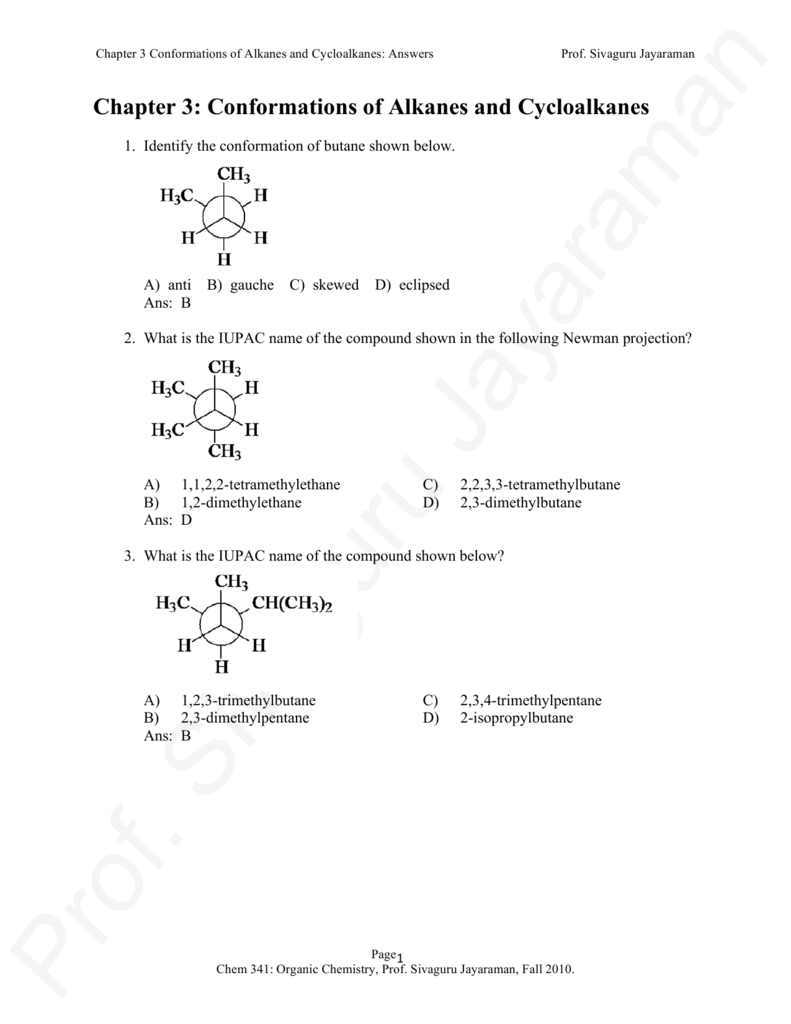
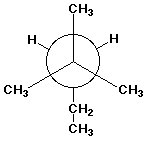
2 3 Dimethylpentane Newman Projection
2 3 dimethylpentane c3 c4 front carbon is c3 sterics f.
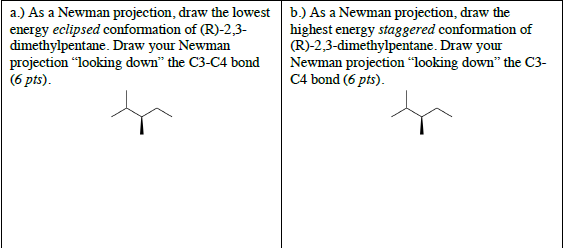
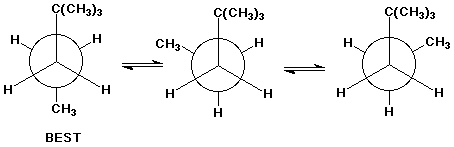
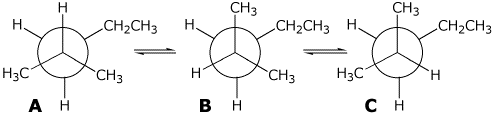
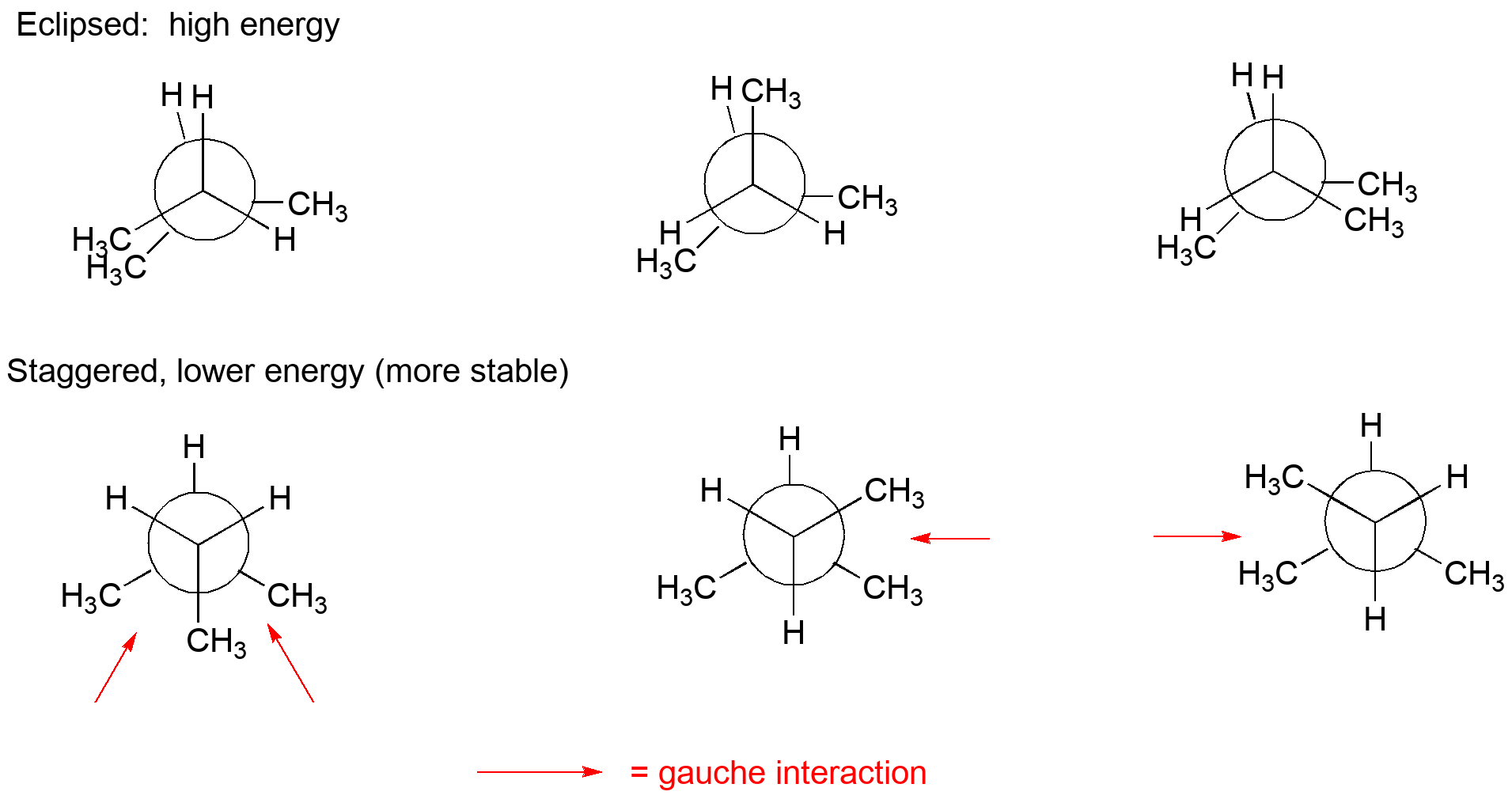
2 3 dimethylpentane newman projection. Start with a circle. Iftwo conformations are equal in energy give them the same number. You will also find the gauche and anti conformations of newman projections. Draw newman projections for the three possible staggered conformers viewing down the c3 c4 bond.
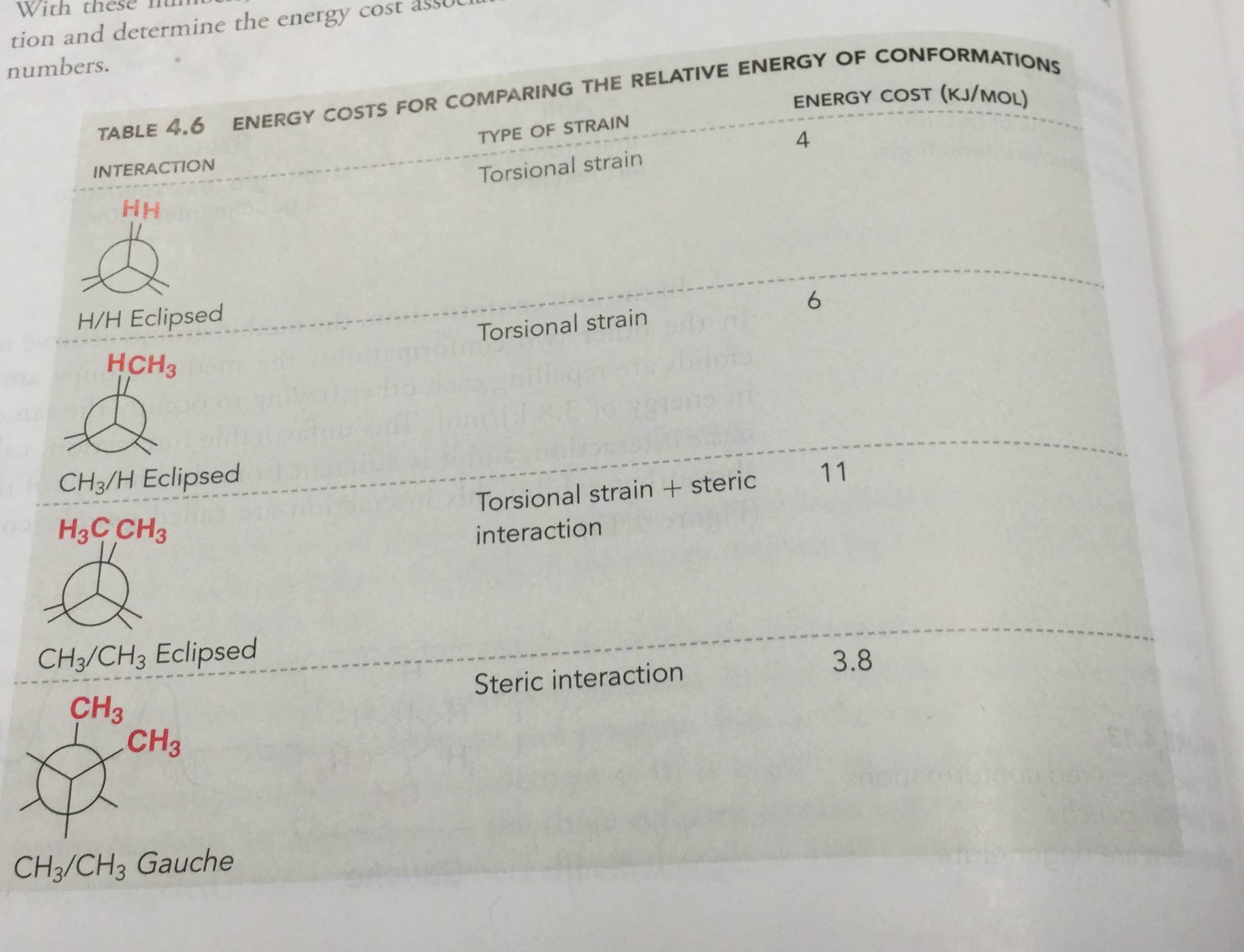
Make sure to note the placement of what is 180 degrees away from the ch 2 ch 3 group. The rear carbon is represented with red color. Newman projections more practice answer key i. Rank the newman projections from 1 lowest energy most stable to 3 highest energy least stable.
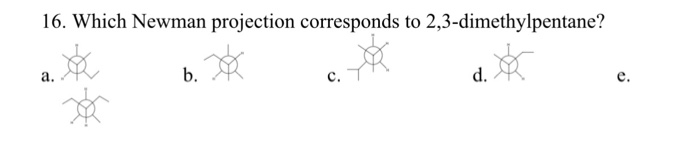
Put 3 bonds on the front that are equally spaced around the circle. What does the newman projection for 2 3 dimethylpentane look like please upload pic. For each of the following draw the best most stable and worst least stable newman projection relative to the bond indicated in each question. Consider the conformations of 2 2 3 trimethylpentane.
Explain why the view down the c3 c4 bond is more informative in identifying the best conformation. Select the most stable conformation. 15 points write newman projections for all three staggered conformations of 2 2 dimethylpentane looking down the c3 c4 bond. Below is the ball and stick model of 2 3 dimethyl pentane.
Also write one newman diagram looking down the c2 c3 bond. If it is a h then it is anti. Take the c 2 carbon as front carbon atom and c 3 as a rear carbon atom. The structure of 2 2 dimethylbutane is as follows.
If possible could you also explain how to calculate the rotation of the c2 c3 bond. Thus the most stable conformation of 2 2 dimethylbutane is.