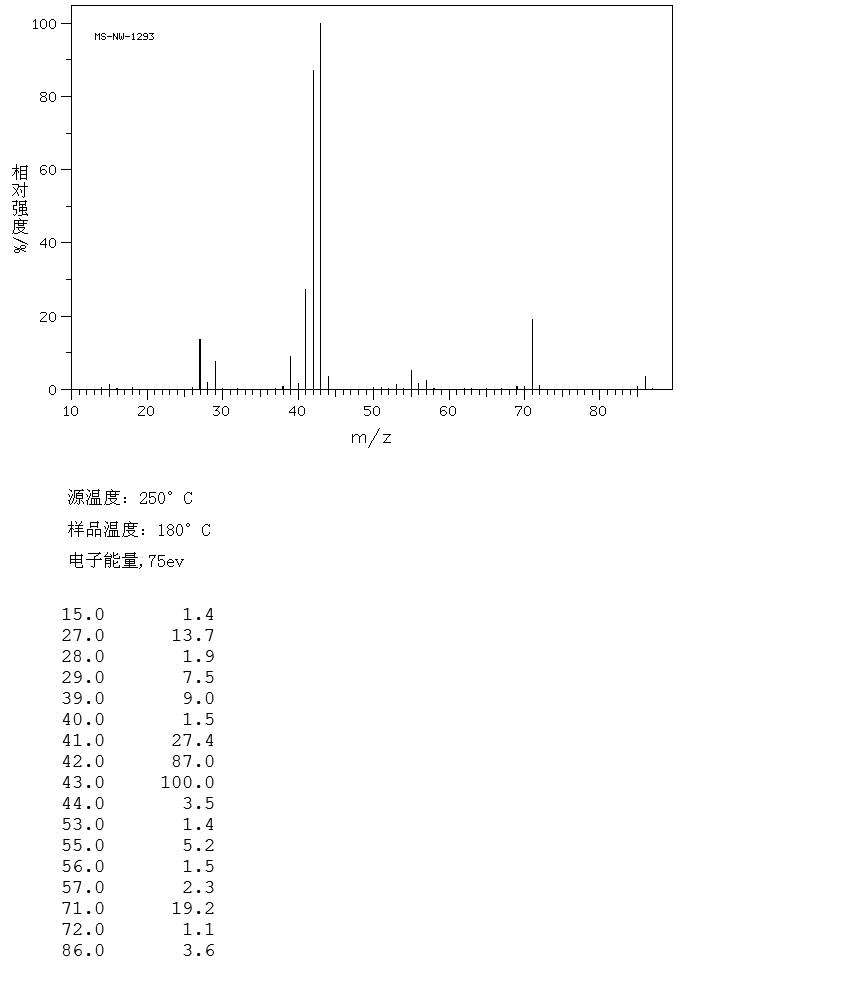
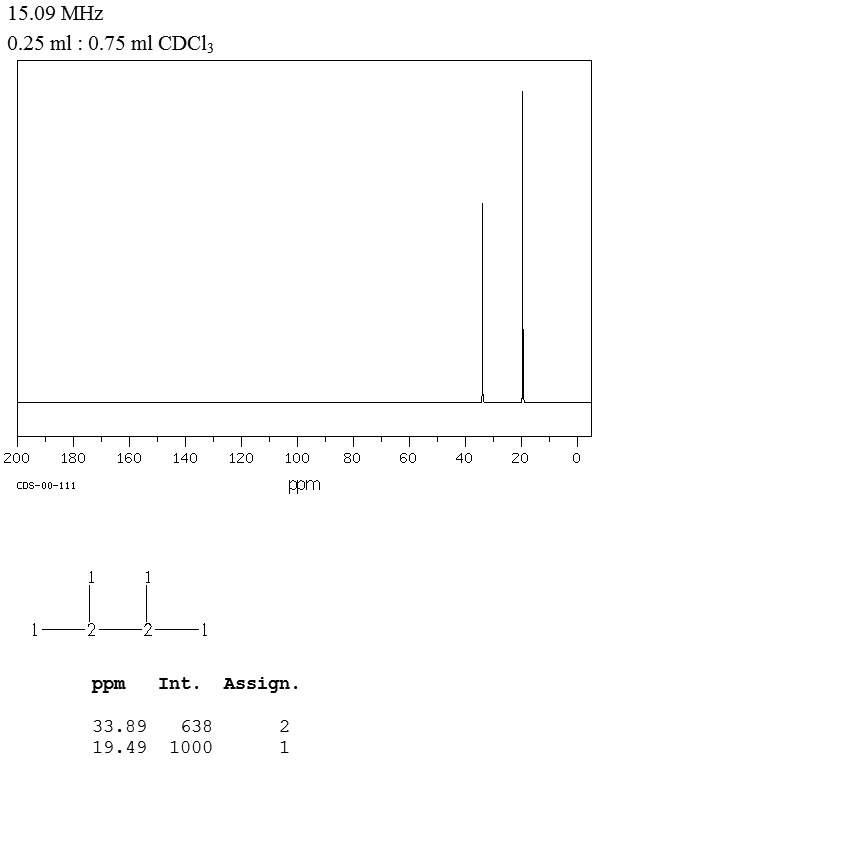
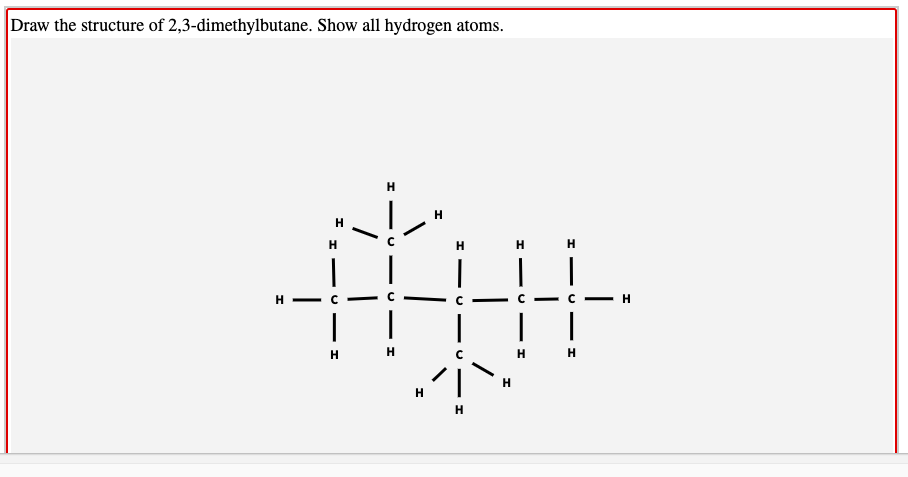
2 3 Dimethylbutane
It is a colorless liquid which boils at 57 9 c.
2 3 dimethylbutane. It certainly does contain a carbon attached to four different groups. General description 2 3 dimethylbutane is a chemical which can undergo photo oxidation with nitrogen oxides to give carbon monoxide and acetone as the major products. 2 3 dimethylbutane appears as a clear colorless liquid with a petroleum like odor. Bioaccumulation estimates from log kow bcfwin v2 17.
1 48 expkow database volatilization from water. 2 3 dimethylbutane chebi 141560 has parent hydride butane chebi 37808 2 3 dimethylbutane chebi 141560 is a alkane chebi 18310 2 3 dimethylbutane chebi 141560 is a volatile organic compound chebi 134179. Stereoisomers of more complex molecules the meso compound c of 2 3 dimethylbutane. 2 3 dimethylbutane is an isomer of hexane.
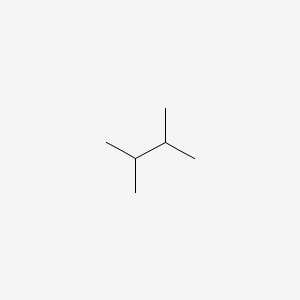
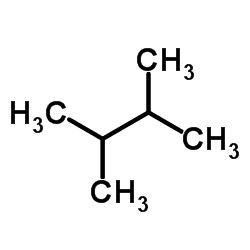
The indicated carbon c 2 is attached to hydrogen a methyl group a chlorine and the rest of the molecule. It has the chemical formula ch3 2chch ch3 2. 2 3 dimethylbutane 2 3 diamine c6h16n2 cid 123390 structure chemical names physical and chemical properties classification patents literature biological. Less dense than water and insoluble in water.
4 01 00 01727 beilstein 464 07 3 rn more. 2 3 dimethylbutane is an isomer of hexane. It has the chemical formula ch 3 2 chch ch 3 2. Vapors heavier than air.
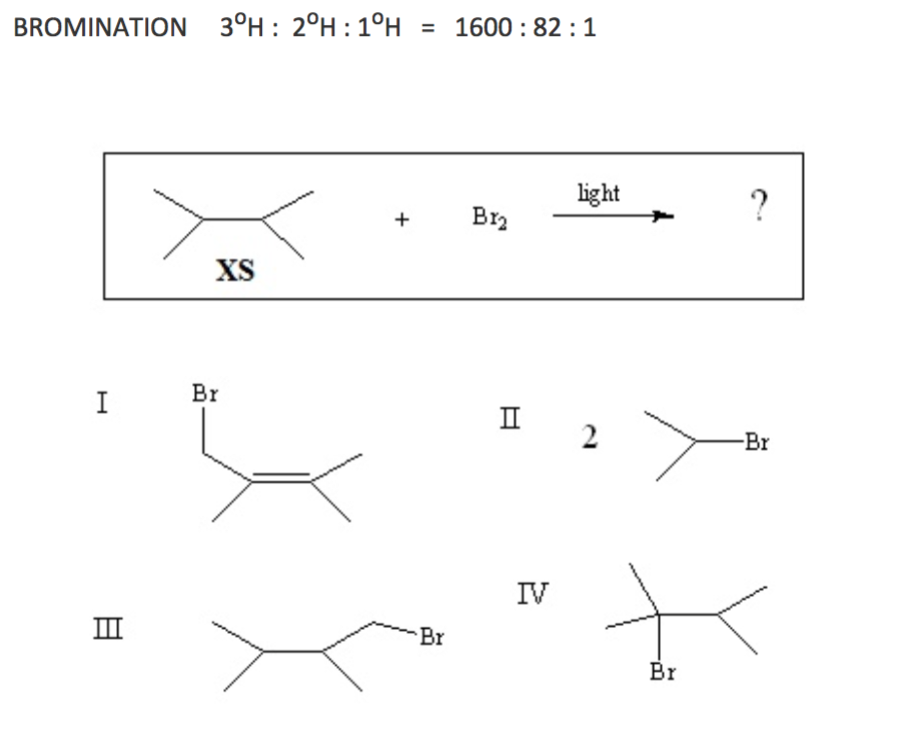
Saturated aliphatic hydrocarbons such as 2 3 dimethylbutane may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. It is a colorless liquid which boils at 57 9 c. General description 2 3 dimethylbutane can can undergo hydrogenolysis over supported catalysts of ruthenium nickel cobalt and iron to form smaller hydrocarbons mainly methane.
Validated by experts validated by.