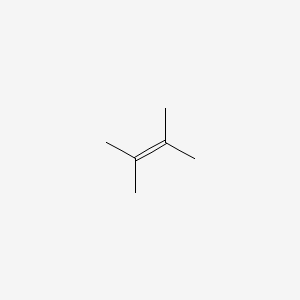
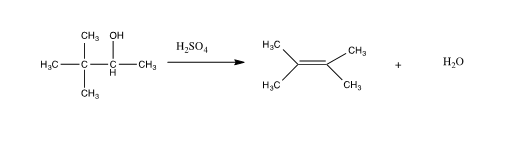
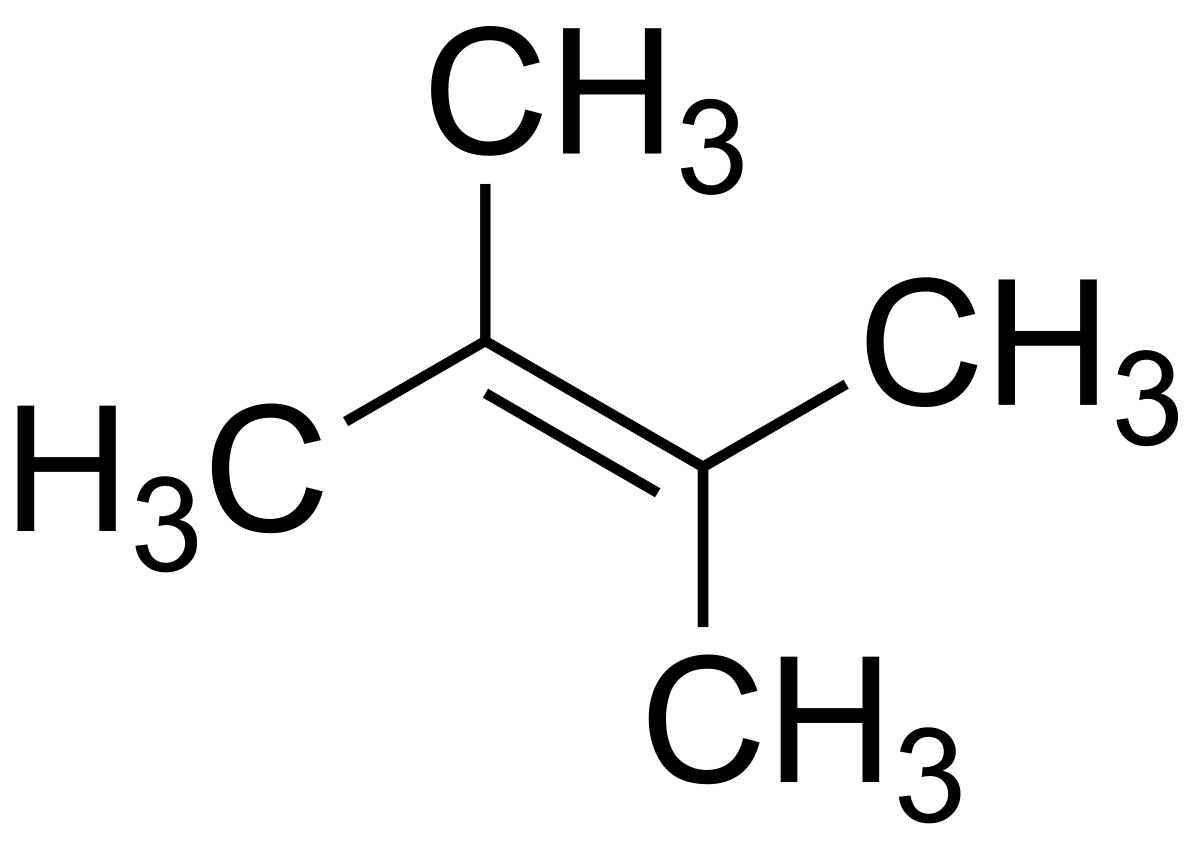
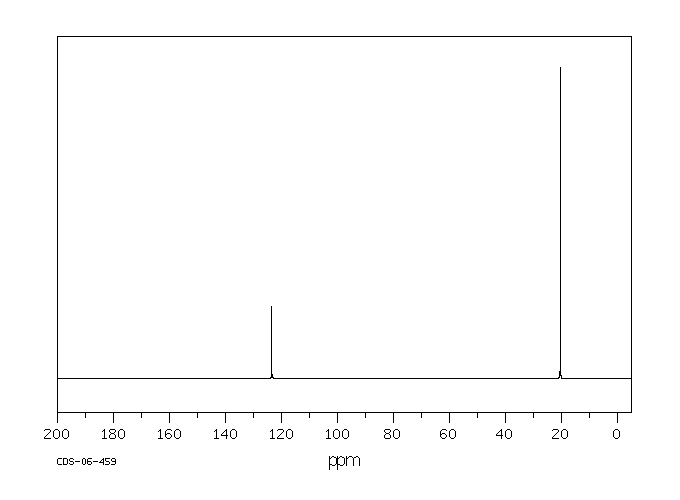
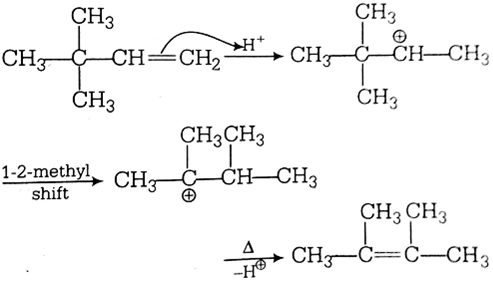
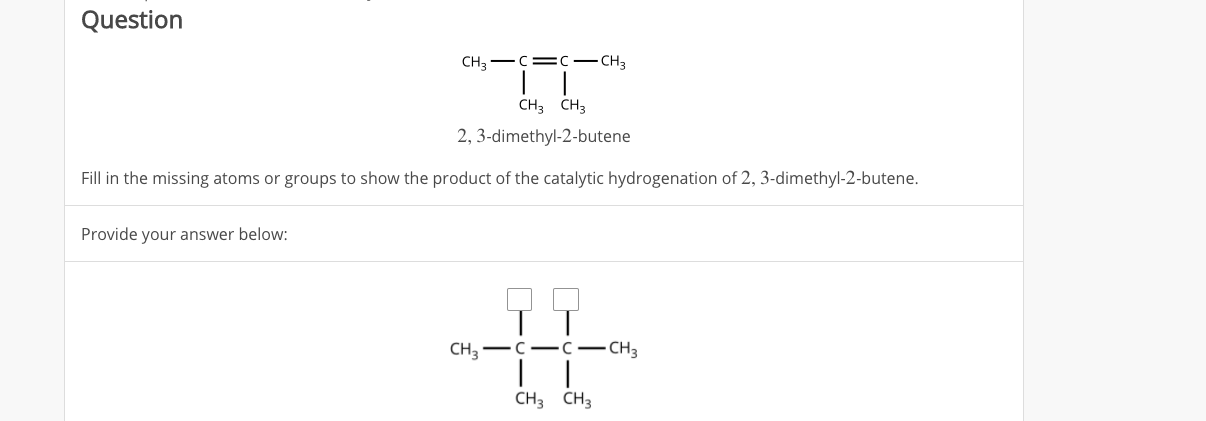
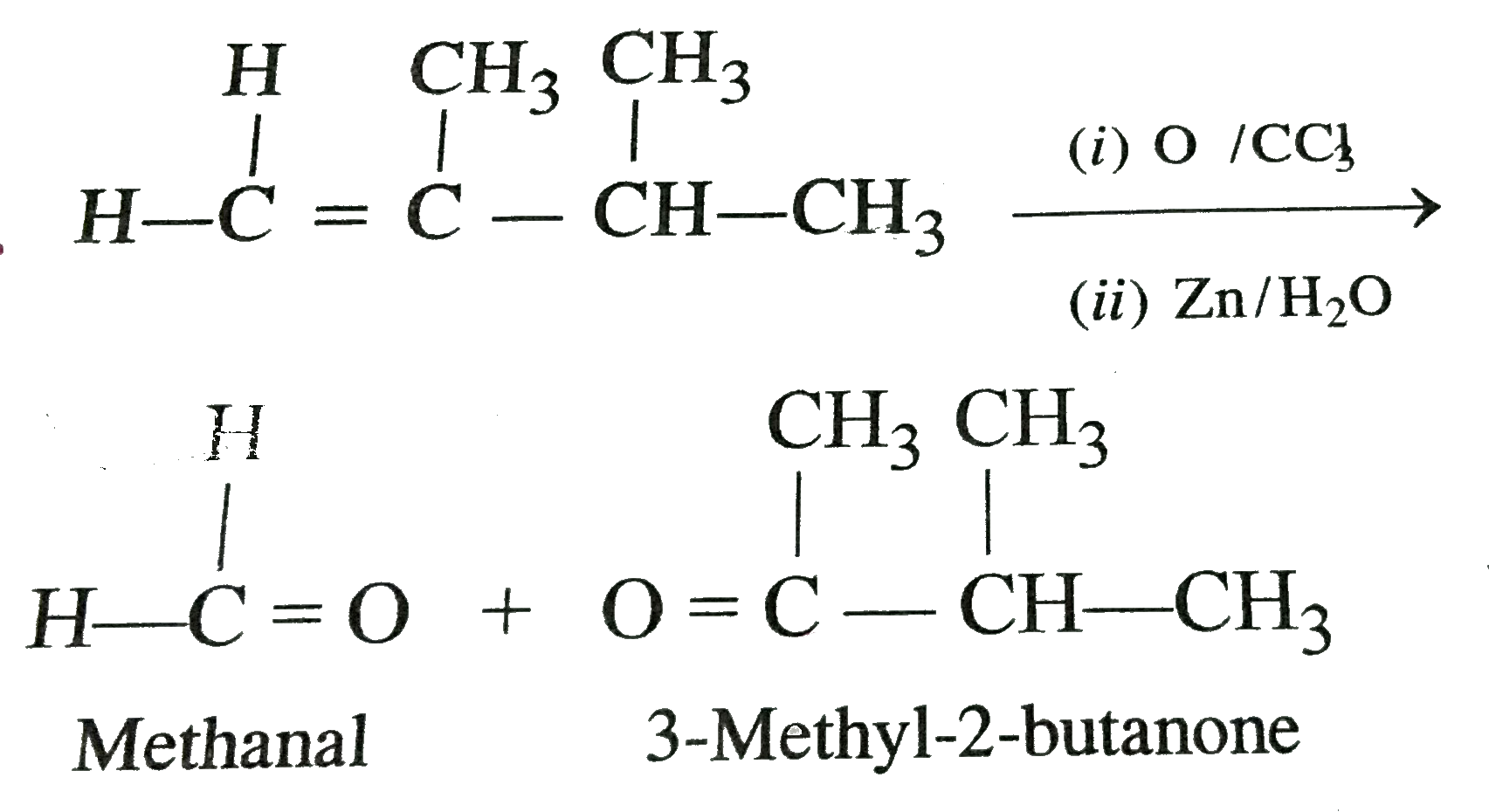
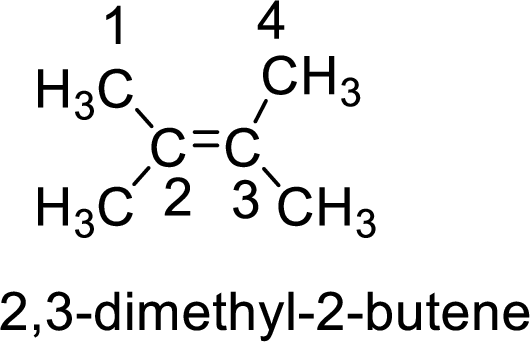
2 3 Dimethyl 2 Butene
Gas phase reaction of 2 3 dimethyl 1 butene with the oh radical has been investigated in the presence of no.
2 3 dimethyl 2 butene. Application 2 3 dimethyl 1 butene was used to investigate the mechanism of the sulfur vulcanization of rubber. It is a colorless liquid and has a slight peppermint or camphor odor. It is colorless liquid which served an important role in the early history of synthetic rubber. Dmb forms adduct with thianthrene cation radical tetrafluoroborate at 0 c and 15 c.
From wikipedia the free encyclopedia pinacolone 3 3 dimethyl 2 butanone is an important ketone in organic chemistry. Gas phase reaction of amylene with the no3 radical concn ranging from 3 8 4 2x10 13 molec cu cm produced acetaldehyde acetone 2 2 3 trimethyloxirane 3 methyl 3 nitroxy 2 butanone and 3 methyl 2 butanone. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. Computed by cactvs 3 4 6 11 pubchem release 2019 06 18 heavy atom count.
Select up to 4 products. It was also found that the percent distribution of products was highly dependent upon pressure 5. 2 3 dimethylbutane s production and use as a high octane fuel and in organic synthesis may result in its release to the environment through various waste streams. It is now a specialty reagent.
Packaging 5 g in glass bottle. If released to soil 2 3 dimethylbutane will have low mobility. Please select more than one item to compare. Computed by pubchem 2 1 pubchem release 2019 06 18 monoisotopic mass.
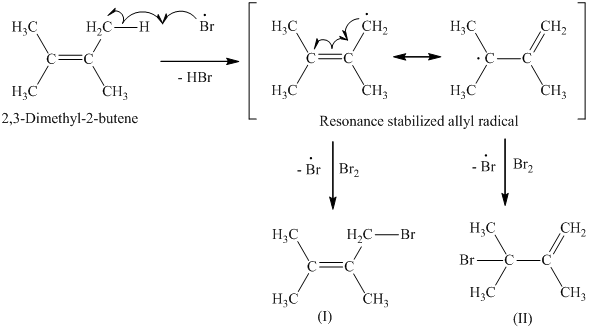
Dimethylbutadiene formally referred to as 2 3 dimethyl 1 3 butadiene is an organic compound with the formula ch 3 2 c 4 h 4. 2 3 dimethyl 2 butene undergoes ozonolysis in dark to yield hydroxyl radical. Reaction of ozone with 2 3 dimethyl 2 butene dmb has been investigated using a flow tube interfaced to uv photoelectron spectrometer. 2 3 dimethylbutane is released into the atmosphere from auto biomass combustion and gasoline vapor emissions.